Though an essential gateway to the liver, NTCP had not been well described until now. Na+-taurocholate co-transporting polypeptide (NTCP) is a protein located exclusively in the membrane of liver cells that enables recycling of bile acid molecules. It is also the cellular receptor of human hepatitis B and D viruses (HBV/HDV). A better understanding of NTCP could enable the development of treatments specifically designed for the liver, and to fight HBV and HDV infection.
Credit: © Kapil Goutam/Nicolas Reyes/CNRS
Though an essential gateway to the liver, NTCP had not been well described until now. Na+-taurocholate co-transporting polypeptide (NTCP) is a protein located exclusively in the membrane of liver cells that enables recycling of bile acid molecules. It is also the cellular receptor of human hepatitis B and D viruses (HBV/HDV). A better understanding of NTCP could enable the development of treatments specifically designed for the liver, and to fight HBV and HDV infection.
NTCP is a difficult protein to study. It weighs only 38 kilodaltons (kDa)1, whereas cryo-electron microscopy, the technology used to study this type of molecule, only works for molecules weighing more than 50 kDA. The challenge was therefore to “enlarge” and stabilise it.
To do this, teams from French and Belgian laboratories2 developed and tested a collection of antibody fragments targeting NTCP. The 3D structures of the resulting complexes were determined using cryo-electron microscopy, and different antibody fragments stabilised and revealed several forms of NTCP.
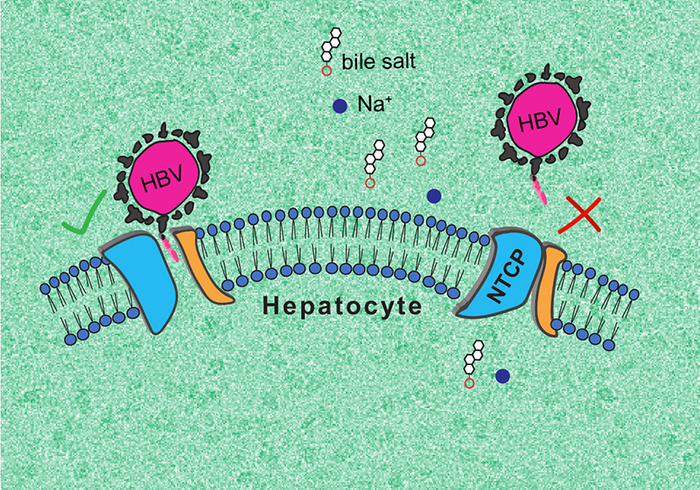
The research team was able to describe two essential NTCP conformations: one in which the protein opens a large membrane pore to bile salts, to which HBV and HDV can bind, and a second, ‘closed’ conformation, that prevents recognition by the viruses.
The first, ‘open’ conformation is very surprising, as no other known molecular transporter forms such a ‘wide open’ pore. In turn, the second conformation could help finding antiviral molecules that prevent HBV and HDV infection. The research team intends to continue its work to fully elucidate the functioning of NTCP.
Footnotes
1- One dalton is one-twelfth the mass of a carbon-12 atom (the mass of a hydrogen atom, approximately).
2- The study was conducted by teams at the MPF Laboratory (Microbiologie fondamentale et pathogénicité) (CNRS/University of Bordeaux), the Membrane Protein Mechanisms Unit at the Institut Pasteur, and the VIB-VUB Center for Structural Biology. This study was supported by the ANRS Emerging Infectious Diseases Program, among others.
Journal
Nature
DOI
10.1038/s41586-022-04723-z
Method of Research
Experimental study
Article Title
Structural basis of sodium-dependent bile salt uptake into the liver
Article Publication Date
11-May-2022