It’s well understood that populations of species don’t distribute at random. Rather, as populations grow, individuals are organized around barriers in the landscape. This organization can be seen in, for example, the growth of the cells around the outer layer of plants and how bacteria arrange themselves in microspores in soil. In both these cases, barriers impact the underlying genetic diversity of the populations. These dynamics have been well researched in larger species—from the way plants disperse to how barnacles spread across a rock, but they have not before been thoroughly studied in smaller systems, like that of bacteria.
Credit: OIST
- It’s well understood that the presence of barriers can impact the underlying genetic diversity of a population, but these dynamics have not been thoroughly studied in small systems, like that of bacteria.
- Now, by combining theoretical models with experiments, scientists have looked closely at how populations of two genetically distinct strains of the bacteria, Escherichia coli, grow in a channel.
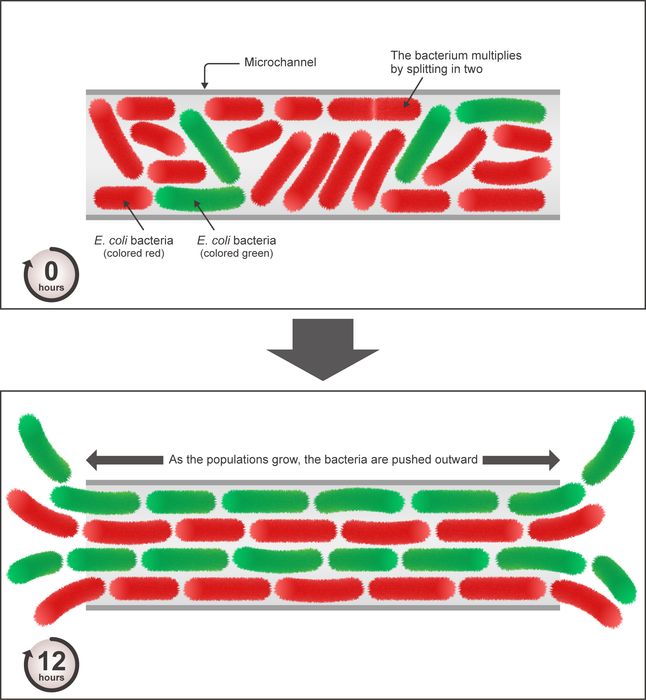
- To observe the population structure, the two strains had different fluorescence—one was red and the other, green.
- The scientists found that, as the populations grew, the bacteria formed lanes of genetically similar individuals that ran parallel to the barriers, resulting in striking color patterns.
- The scientists highlighted that as other biological systems present similar structures—for example, bacteria in soil and cells growing in certain body tissues—these findings can have implications for a range of research.
It’s well understood that populations of species don’t distribute at random. Rather, as populations grow, individuals are organized around barriers in the landscape. This organization can be seen in, for example, the growth of the cells around the outer layer of plants and how bacteria arrange themselves in microspores in soil. In both these cases, barriers impact the underlying genetic diversity of the populations. These dynamics have been well researched in larger species—from the way plants disperse to how barnacles spread across a rock, but they have not before been thoroughly studied in smaller systems, like that of bacteria.
Now, by combining theoretical models and experiments, scientists from the Biological Complexity Unit and the Micro/Bio/Nanofluidics Unit at the Okinawa Institute of Science and Technology Graduate University (OIST) have shown that, when constrained to a channel, the bacteria Escherichia coli will form lanes of genetically similar individuals that run parallel to the barriers. This study was published in PNAS.
“If populations grow in the presence of spatial barriers, the barriers can constrain the movement of individuals and affect the evolution of a population,” explained first author Ms. Anzhelika Koldaeva, PhD candidate in the Biological Complexity Unit. “We found that in a channel, the bacteria tend to align along the barriers and form patterns in their populations. Other biological systems present similar structures—for example, bacteria in porous soil and cells growing in certain body tissues—so these findings can have implications for a range of research.”
Escherichia coli, also known as E. coli, are rod-shaped, single-celled bacteria that are found in many different environments, including the food and intestines of healthy people and animals. E. coli reproduce asexually with a “mother” cell splitting apart to create two “daughter” cells. To observe the population structure, two strains of E. coli were used, which had different fluorescence—one was red and the other, green. This way, the researchers could identify which daughter came from which mother. The two strains were the same in terms of size, the length of the reproductive cycle, and other measures of fitness.
Researchers from the Biological Complexity Unit first developed a model for the dynamics of the colony. They simulated the growth of the populations over several generations with the aim of experimental validations as the next step. Then, the Micro/Bio/Nanofluidics Unit teamed up with them and took on the experimental challenge.
“We created a microfluidic platform with a temperature and humidity control, which contained tiny microchannels to house the bacteria,” explained Prof. Amy Shen, Principle Investigator of the Micro/Bio/Nanofluidics Unit. “This was very difficult and a lot more complicated than a standard cell experiment. We had to feed bacteria and the system was susceptible to contamination.”
This process was so challenging that it took former OIST PhD student Dr. Paul Hsieh-Fu Tsai (now an Assistant Professor at Chang Gung University in Taiwan) almost a year to build a reliable platform for long-term imaging of the bacterial growth. For the experiments, individual bacteria from each strain were placed approximately in the center of the microchannel and videos were recorded over an 80-hour period to observe the patterns that formed. These videos were then analyzed and the growth dynamics from these experiments was compared to the simulations.
Both the simulations and the experiments confirmed that, within a few generations, in the first 12 hours, the two strains of bacteria started to form the distinctive lane-like patterns. E. coli are elongated and thus aligned themselves parallel to the sides of the microchannel. However, the two different strains did not become mixed but rather, as time went on, they became more segregated into their own lanes.
Prof. Simone Pigolotti, Principle Investigator of the Biological Complexity Unit, concluded, “by using a combination of theory and experiments, we found something unexpected in the E. coli system, which is used by a lot of researchers across the world.”
Journal
Proceedings of the National Academy of Sciences
DOI
10.1073/pnas.2120821119
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Population genetics in microchannels
Article Publication Date
18-Mar-2022




