A single protein can reverse the developmental clock on adult brain cells called astrocytes, morphing them into stem-like cells that produce neurons and other cell types, UT Southwestern researchers report in a PNAS study. The findings might someday lead to a way to regenerate brain tissue after disease or injury.
Credit: UT Southwestern Medical Center
A single protein can reverse the developmental clock on adult brain cells called astrocytes, morphing them into stem-like cells that produce neurons and other cell types, UT Southwestern researchers report in a PNAS study. The findings might someday lead to a way to regenerate brain tissue after disease or injury.
“We’re showing that it may be possible to reprogram the fate of this subset of brain cells, giving them the potential to rebuild the damaged brain,” said study leader and co-corresponding author Chun-Li Zhang, Ph.D., Professor of Molecular Biology and an Investigator in the Peter O’Donnell Jr. Brain Institute.
During development, mammalian stem cells readily proliferate to produce neurons throughout the brain and cells – called glia – that help support them. Glia help maintain optimal brain function by performing essential jobs like cleaning up waste and insulating nerve fibers. However, the mature brain largely loses that stem cell capacity. Only two small regenerative zones, or niches, remain in the adult brain, Dr. Zhang explained, leaving it with extremely limited capacity to heal itself following injury or disease.
Recent research has suggested that glia can be prompted to produce neurons in some models of brain injury or after genetic manipulation. Although these findings are promising, regenerating healthy brain tissue will require production of multiple cell types, rather than only neurons, said Dr. Zhang.
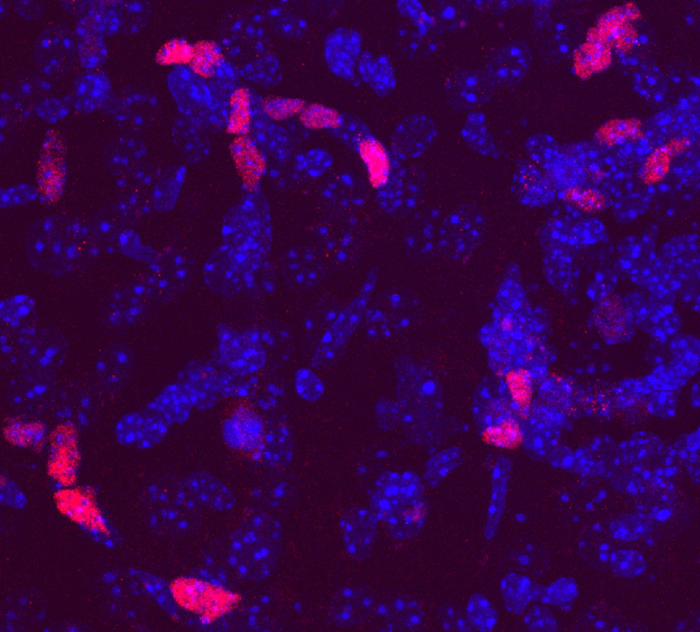
Looking for a way to spur this “multipotent” regeneration, Dr. Zhang and his colleagues used a genetic engineering technique in adult mouse brains to induce astrocytes, a subset of glia, to produce different transcription factors, proteins pivotal for controlling cell identity. These experiments showed that a single transcription factor – a protein known as DLX2 – appeared to reprogram astrocytes into neural stem-like cells capable of producing neurons and multiple subtypes of glial cells.
The researchers confirmed these findings both using a technique called lineage tracing, in which they followed progeny of the altered astrocytes as they multiplied, as well as marker analysis that showed that these new cells had the expected identities of neurons or glia. Working with the team of co-corresponding author Gary Hon, Ph.D., Assistant Professor of Obstetrics and Gynecology and in the Cecil H. and Ida Green Center for Reproductive Biology Sciences and the Lyda Hill Department of Bioinformatics, global gene expression analysis showed that prompting astrocytes to produce DLX2 appeared to reprogram them into stem-like cells with features of both immature brain cells found earlier in development and cells found in the regenerative niches of the adult brain.
Dr. Zhang and his colleagues suggest that DLX2 might someday be used as a tool to treat traumatic brain injuries, strokes, and degenerative conditions such as Huntington’s disease. Researchers in the Zhang lab are planning to study this approach in animal models.
Current UT Southwestern researchers who contributed to this study include Sergio Cananzi, Lei-Lei Wang, and Yuhua Zou. Other participants include co-lead authors Boxun Li, now at Duke University; Yunjia Zhang, now at the Beijing Genomics Institute, China; Chuanhui Han, now at Peking University, China; and Yang-Xin Fu, now at Tsinghua University, China.
This research was supported by funding from The Welch Foundation (I-1724 and I-1926-20170325), the Decherd Foundation, the Texas Alzheimer’s Research and Care Consortium (TARCC2020), the Kent Waldrep Foundation Center for Basic Research on Nerve Growth and Regeneration, the National Institutes of Health (NS099073, NS092616, NS111776, NS117065, NS088095, DP2GM128203, and UM1HG011996), the Cancer Prevention Research Institute of Texas (CPRIT) (RR140023 and RP190451), the Department of Defense (PR172060), the Burroughs Wellcome Fund (1019804), the Harold C. Simmons Comprehensive Cancer Center, and the Green Center for Reproductive Biology.
Dr. Zhang is a W.W. Caruth, Jr. Scholar in Biomedical Research at UT Southwestern. Dr. Hon is a CPRIT Scholar.
About UT Southwestern Medical Center
UT Southwestern, one of the nation’s premier academic medical centers, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty has received six Nobel Prizes and includes 25 members of the National Academy of Sciences, 17 members of the National Academy of Medicine, and 14 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 2,800 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than 117,000 hospitalized patients, more than 360,000 emergency room cases, and oversee nearly 3 million outpatient visits a year.
Journal
Proceedings of the National Academy of Sciences
Article Publication Date
7-Mar-2022




