Researchers at the NYU Pain Research Center have identified a mechanism that underlies inflammation and pain in the colon, and demonstrated that blocking a key receptor from entering colon cells can inhibit inflammation and pain, uncovering a potential target for treating pain in inflammatory bowel disease.
Credit: Bunnett Lab, NYU Dentistry
Researchers at the NYU Pain Research Center have identified a mechanism that underlies inflammation and pain in the colon, and demonstrated that blocking a key receptor from entering colon cells can inhibit inflammation and pain, uncovering a potential target for treating pain in inflammatory bowel disease.
Their study, published in the Proceedings of the National Academy of Sciences (PNAS), was conducted in mice with colitis, an inflammatory disease marked by chronic and sometimes painful inflammation of the large intestine.
The digestive tract is home to a large number of proteases, or enzymes that break down proteins. These proteases come from a variety of sources, including the microbiota, inflammatory cells, or digestive enzymes in the intestine.
While proteases are important for digestion and help to degrade proteins in the gut, many also signal cells by activating specific G protein-coupled receptors (GCPRs). GCPRs are a large family of receptors that regulate many processes in the body and are the target of one third of clinically used drugs. When proteases activate one such GCPR—protease-activated receptor-2, or PAR2—on nerve cells, it causes the release of mediators that produce pain.
Studies show that protease activation of PAR2 is involved in gastrointestinal diseases that can be associated with pain, including inflammatory bowel disease, irritable bowel syndrome, and cancer. But until now, scientists have not fully understood the receptor’s signaling mechanism and how it induces pain.
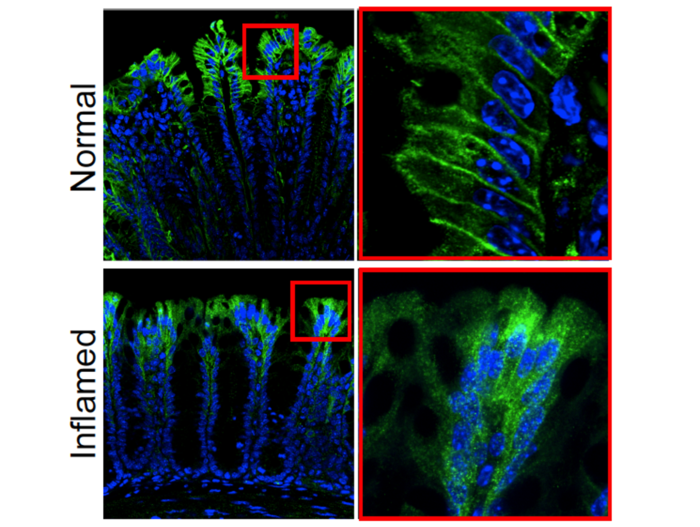
To pinpoint PAR2’s location in the gut, the researchers created a mouse model in which the gene for PAR2 is fused to a green fluorescent protein. When a cell expresses PAR2, it lights up green, allowing the researchers to precisely see where the receptor is positioned. They found that PAR2 was very highly expressed on the surface or membrane of the epithelial cells that line the small and large intestines, and to a lesser extent in nerve fibers in these areas.
The researchers then discovered a key difference in the location and behavior of PAR2 in healthy mice versus mice with colitis. In healthy mice, PAR2 was found on the membrane of colonic epithelial cells, but in mice with colitis, it shifted from the surface of cells to compartments within cells called endosomes. When the receptor moved into endosomes, it generated signals that cause inflammation and pain by disrupting the normal protective function of cells lining the colon.
“We identified not only where this receptor is in the digestive tract, but also how it signals inflammation and pain in the colon,” said Nigel Bunnett, PhD, professor and chair of the Department of Molecular Pathobiology at NYU College of Dentistry and the study’s senior author. “This more complete understanding of PAR2 and its signaling mechanism could ultimately help us to better treat inflammatory and painful diseases of the colon.”
Additional studies using human colon tissue confirmed that activating PAR2 induces inflammation in the colon.
If PAR2 moving from the surface of cells into endosomes leads to inflammation and pain, could blocking the receptor from entering cells limit inflammation and pain? To test this idea, the researchers prevented the movement of PAR2 into cells by knocking down the expression of a protein called dynamin-2. Keeping the receptor out of cells did, in fact, inhibit signaling and significantly reduced pain and inflammation.
The findings suggest that PAR2—and specifically, PAR2 in endosomes—may be a useful target in treating pain in inflammatory bowel disease.
“This could be achieved through blocking PAR2 from entering cells, as we did in this study by inhibiting dynamin-2,” said Bunnett. “It could also mean getting drugs that activate PAR2 not just to the surface of cells, but into the interior of cells using nanoparticles to reach the receptor in endosomes.”
The study was a collaboration between researchers at the NYU Pain Research Center at NYU College of Dentistry, Monash University in Australia, and the University of California, Los Angeles. The research was supported by grants from the National Institutes of Health (NS102722, DE026806, DK118971, and DE029951), U.S. Department of Defense (W81XWH1810431), Australia’s National Health and Medical Research Council (63303), and the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology. Gastrointestinal research in Bunnett’s lab is also supported by Takeda Pharmaceuticals.
About NYU College of Dentistry
Founded in 1865, New York University College of Dentistry (NYU Dentistry) is the third oldest and the largest dental school in the US, educating nearly 10 percent of the nation’s dentists. NYU Dentistry has a significant global reach with a highly diverse student body. Visit dental.nyu.edu for more.
Journal
Proceedings of the National Academy of Sciences
DOI
10.1073/pnas.2112059119
Subject of Research
Animals
Article Title
Mice expressing fluorescent PAR reveal that endocytosis mediates colonic inflammation and pain
Article Publication Date
4-Feb-2022
COI Statement
N.W.B. is a founding scientist of Endosome Therapeutics, Inc. Research in the laboratories of N.W.B., N.A.V., and D.P.P. is funded in part by Takeda Pharmaceuticals International.




