Proteins are the building blocks of all living things. A vast amount research takes place on how these proteins are made and what they do, from enzymes that carry out chemical reactions to messengers that transmit signals between cells. In 2004, Aaron Ciechanover, Avram Hershko, and Irwin Rose won the Nobel Prize in Chemistry for a different but just as important process of protein machinery: how organisms break down proteins when they are finished doing their job.
Credit: University of Chicago
Proteins are the building blocks of all living things. A vast amount research takes place on how these proteins are made and what they do, from enzymes that carry out chemical reactions to messengers that transmit signals between cells. In 2004, Aaron Ciechanover, Avram Hershko, and Irwin Rose won the Nobel Prize in Chemistry for a different but just as important process of protein machinery: how organisms break down proteins when they are finished doing their job.
Protein degradation is a carefully orchestrated process. Proteins are marked for disposal with a molecular label called ubiquitin, and then fed into proteasomes, a kind of cellular paper shredder that chops up the proteins into small pieces. This process of ubiquitination, or labeling proteins with ubiquitin, is involved in a wide range of cellular processes, including cell division, DNA repair, and immune responses.
In a new study published in Nature on November 17, 2021, researchers from the University of Chicago used advanced electron microscopes to delve deeper into the process of protein degradation. They described the structure of a key enzyme that helps mediate ubiquitination in yeast, part of a cellular process called the N-degron pathway that may be responsible for determining the rate of degradation for up to 80% of equivalent proteins in humans. Malfunctions in this pathway can lead to accumulation of damaged or misfolded proteins, which underlies the aging process, neurodegeneration, and some rare autosomal recessive disorders, so understanding it better provides an opportunity to develop treatments.
Minglei Zhao, PhD, Assistant Professor of Biochemistry and Molecular Biology, and his colleagues studied an E3 ligase—a type of enzyme that helps join larger molecules together—called Ubr1. In baker’s yeast, Ubr1 helps initiate the ubiquitination process as it attaches ubiquitin to proteins and elongates it into a chain of molecules known as a polymer. Polymers, which are more commonly known as the building blocks of synthetic materials like plastics, also occur naturally when large molecules (in this case ubiquitin) are connected in repeating subunits.
“Until this study, we didn’t know that much about how ubiquitin polymers are structurally formed,” Zhao said. “Now we are starting to get an idea of how it’s first installed onto the protein substrate, and then how the polymers are formed in a linkage-specific manner. This is a milestone in terms of understanding polyubiquitination at a near atomic level.”
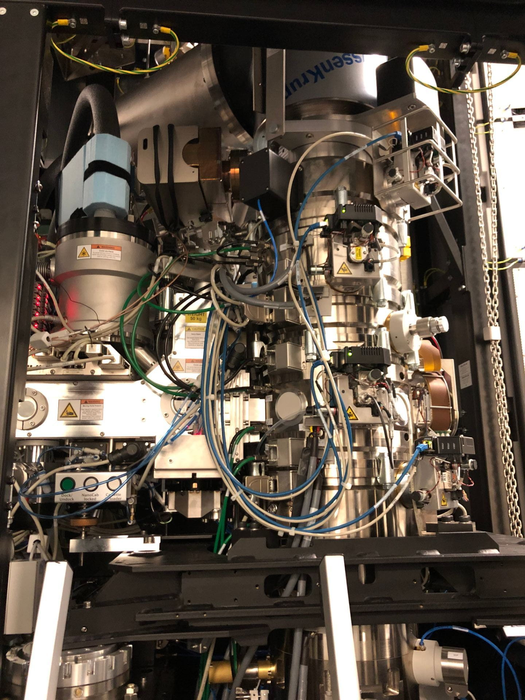
In this study, Zhao and his team used some chemical biology techniques to mimic the initial steps of the process for attaching ubiquitin to proteins. Then, they employed another Nobel Prize-winning innovation called cryo-electron microscopy (cryo-EM) to capture the process. Cryo-EM involves flash-freezing solutions of proteins and then using a powerful electron microscope to produce images of individual molecules or subcellular structures. About 10 years ago, breakthroughs in hardware and software produced microscopes and detectors that could capture molecular images at much higher resolution. In 2017, Jacques Dubochet, Joachim Frank, and Richard Henderson won the Nobel Prize in Chemistry for developing cryo-EM techniques, which allow researchers to create a snapshot that literally freezes “live” action of a biological process.
Zhao’s team took advantage of a $10 million investment by the Biological Sciences Division in the Advanced Electron Microscope Facility to use cryo-EM to study ubiquitination in more detail. They were able to describe the structure of several intermediate enzyme complexes involved in the pathway, which will help researchers looking for ways to target proteins with drugs or intervene in a malfunctioning protein degradation process.
“Cryo-EM is exciting because after the data processing is done, a new structure pops out that you’ve never seen before,” Zhao said. “Now we can use what we’ve learned and repurpose the enzymes by introducing small molecules or mixture of peptides to degrade the proteins we want.”
Journal
Nature
DOI
10.1038/s41586-021-04097-8
Method of Research
Imaging analysis
Subject of Research
Cells
Article Title
‘Structural insights into Ubr1-mediated N-degron polyubiquitination
Article Publication Date
17-Nov-2021




