Credit: Kanazawa University
[Background]
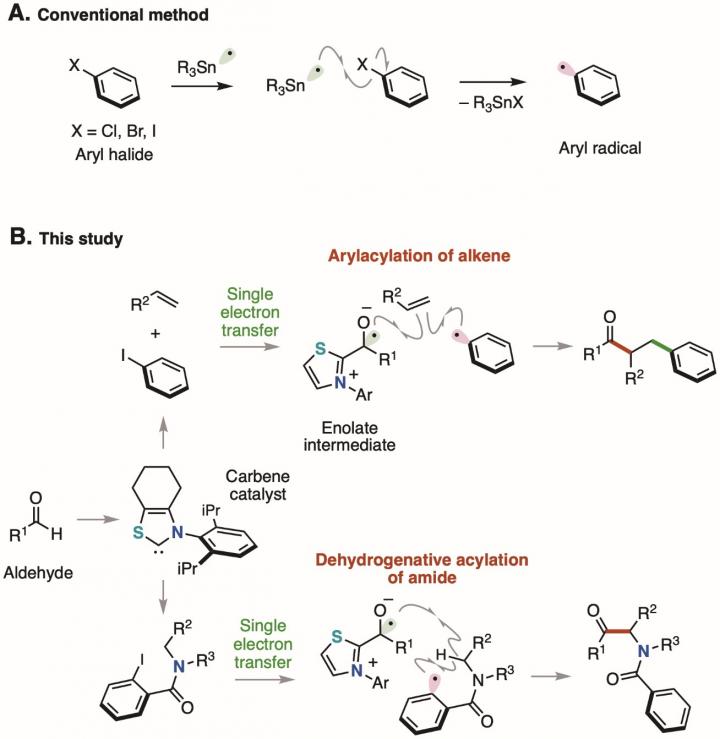
Aryl halides*1) with a benzene ring directly bonded to a halogen atom are readily available and chemically stable, so they are used as a source of benzene rings in organic synthesis. For example, a chemical reaction that generates a highly reactive aryl radical*2) from an aryl halide using a toxic tin compound has long been known as a method for supplying a benzene ring (Figure 1A). In recent years, chemical reactions have been developed, in which an aryl halide is reduced using a metal catalyst or a photocatalyst*3) followed by cleavage of the bond between the benzene ring and the halogen atom to generate an aryl radical. However, since the methods previously reported require metal salts and/or excess amounts of an oxidizing agent or a reducing agent, chemical reactions with less environmental impact are desirable.
[Results]
The research group of Kanazawa University led by Prof. Ohmiya has been developing novel chemical reactions using newly developed metal-free organic catalysts*4) to produce various useful chemicals in a much easier manner than with conventional methods (see EurekAlert! webpages such as https:/
A single electron transfer from an enolate*6) intermediate consisting of a thiazolium-type N-heterocyclic carbene catalyst and an aldehyde to an aryl iodide and the subsequent cleavage of the bond between the benzene ring and the iodine atom generate an aryl radical in a catalytic manner. Considering the oxidation potential of the enolate intermediate (Eox = -0.97 V) and the reduction potential of the aryl iodide (Ered = -2.24 V), single electron transfer from the enolate intermediate to the aryl iodide, i.e. single electron reduction, is thermodynamically unfavorable. However, it is considered that the reaction took place due to kinetic factors because the two reaction steps, i.e. 1) single electron transfer from the enolate intermediate to the aryl iodide and 2) cleavage of the bond between the benzene ring and the iodine atom, proceed rapidly. The aryl radical generated acts as a source of the benzene ring, the difunctionalization of the alkene*7) proceeds, and a benzene-ring substituted ketone is obtained. In addition, by using the aryl radical generated for the intramolecular hydrogen abstraction reaction, the dehydrogenative acylation*8) of the amide proceeds, and an α-aminoketone compound can be obtained. Substrates with various functional groups can be used in these molecular conversion reactions. Derivatives of pharmaceuticals can also be synthesized by the dehydrogenative acylation of amides (Figure 2).
[Future prospects]
The results of this study are the development of a chemical reaction that cleaves the bond between the benzene ring of an aryl halide and the halogen atom by using an organic catalyst that has a low impact on the environment, leading to generation of an aryl radical. Since aryl radicals can be easily generated from aryl halides that are widely used in organic synthesis, this is expected to be a powerful technology for precisely synthesizing medical and agricultural drugs as well as chemical materials.
###
[Glossary]
*1) Aryl halide
A compound in which one of the hydrogen atoms on the aromatic (benzene) ring is replaced with a halogen (F, Cl, Br, I) atom.
*2) Aryl radical
Aryl (benzene ring) compound having unpaired electrons.
*3) Photocatalyst
A catalyst that can perform electron or energy transfer by absorbing light (here, visible light).
*4) Organic catalyst
A catalyst is a chemical compound that facilitates chemical reactions to proceed without itself being changed before and after chemical reactions. An organic catalyst is a low molecular weight organic compound consisting of elements such as carbon, hydrogen, oxygen, nitrogen, sulfur, etc. but without any metal element.
*5) Carbene
A carbene is a divalent chemical species containing a carbon atom with only 6 electrons.
*6) Enolate
Enolates are organic anions derived from the deprotonation of carbonyl compounds.
*7) Alkene
An organic compound with a double bond between two adjacent carbon atoms.
*8) Acylation
A reaction to introduce an acyl group to an organic compound; an acyl group is a derivative of carboxylate deprived of a hydroxyl group.
Media Contact
Ayako Honda
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




