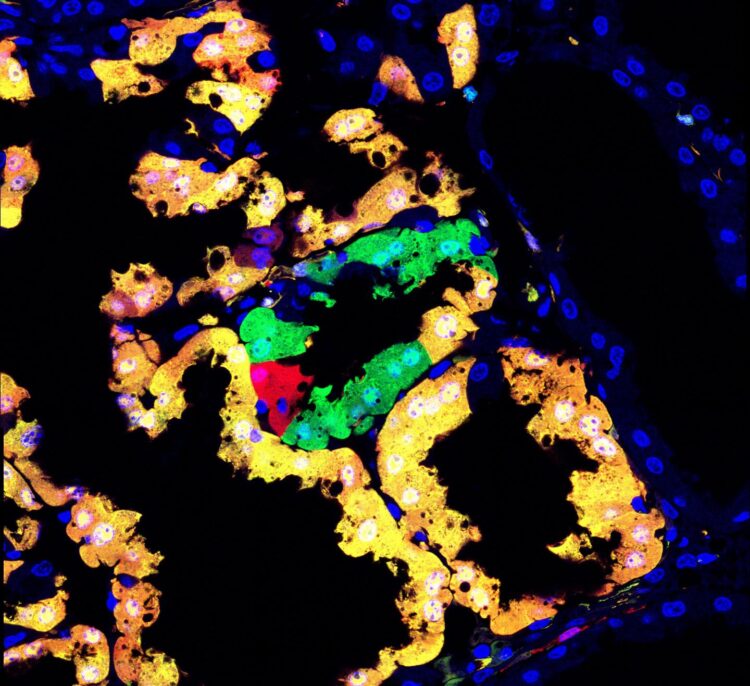
96% of mouse genome accessible to high-resolution single-cell analysis — MADM technology expanded in new resource published in Cell Reports
Genetic mosaic individuals, which contain cells of different genotypes, arise naturally in multicellular organisms. In humans, the development of cancer – where one cell acquires a mutation that allows it to proliferate, while other cells don’t – is a prime example of genetic mosaicism. But inversely, genetic mosaicism can be used to study and understand the development of disease.
A common quirk of nature used to understand genes
Up until now, only about 25 percent of mouse genes could be mutated and followed using the MADM technique, as MADM technology was limited to three of the mice chromosomes. Now, Hippenmeyer and his group at IST Austria have dramatically expanded this resource. The group has successfully placed the “MADM marking cassette” required for the MADM technique on all mouse chromosomes (except the sex chromosomes). Now, more than 96% of genes can be mutated and followed on the single-cell level using MADM. “We can now easily manipulate almost every mouse gene, and subject every gene to high-resolution, phenotypic genetic mosaic analysis”, Hippenmeyer explains.
New avenues for cancer research
Hippenmeyer anticipates that this resource will be a boost to the study of disease and general mechanisms of development. “Now, we can study genes associated with diseases that emerge from a single mutated cell, of which cancer is the prime example. With our resource, researchers can systematically study every single known tumor suppressor gene and its role in cancer development and evolution, including in combination with other mutations.” In recent years, researchers have used MADM in several cancer studies, including for screening for drug targets. “Our MADM library is not only a way to analyze disease progression, but also provides a platform for drug and drug target discovery”, Hippenmeyer adds. “This is not limited to cancer, MADM can also be used to study and understand disease in many contexts including neuro-developmental and other brain disorders, which is a prime interest of the Hippenmeyer group.”
In their paper, Hippenmeyer and his group used the novel resources to expand the MADM application spectrum and shed light on an intriguing problem in biology. They found evidence that chromosome segregation during asymmetric cell division follows a non-random pattern. “Our results indicate for the first time in vivo, that the way how parental chromosomes segregate during stem cell division could instruct the cellular fate of resulting daughter cells. In a broader context these findings are relevant for our general understanding of stem cell biology and perhaps the mechanisms of cancer progression” In future, Hippenmeyer, a neuroscientist, will use the expanded capabilities of MADM to study stem cell behavior during brain development and the mechanisms ensuring that brains develop to the correct size. In humans, disorders of brain size, such as micro- and macroencephaly, are associated with epilepsy and intellectual disability. “We can now ask what goes wrong in a stem cell, so that the brain develops to be too large or too small. We anticipate that our future results can also provide a basis of prospective stem cell-based brain repair and regeneration”.
###
Media Contact
Patrick Müller
[email protected]
Related Journal Article
http://dx.