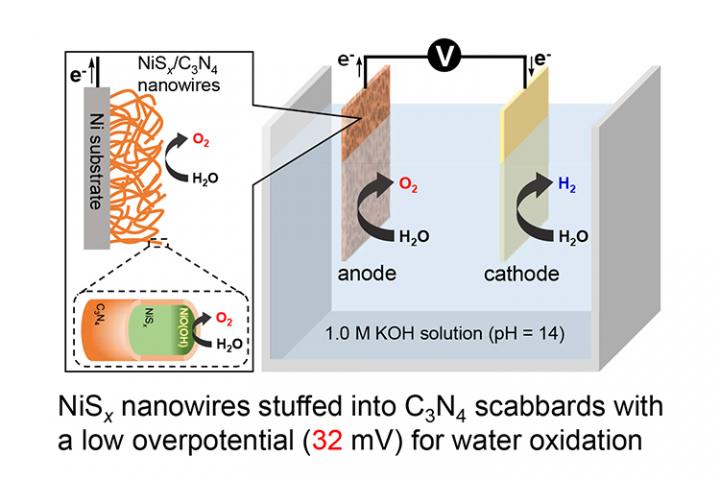
Development of highly efficient nickel sulfide nanowires stuffed into carbon nitride scabbards as anodes with extremely low overpotential for electrocatalytic water splitting applications
Niigata, Japan – In the recent past, there has been a paradigm shift towards renewable sources of energy in order to address the concerns pertaining to environmental degradation and dwindling fossil fuels. A variety of alternative green energy sources such as solar, wind, hydrothermal, tidal etc., have been gaining attention to reduce the global carbon footprints. One of the key challenges with these energy generation technologies is that they are intermittent and are not continuously available.
“We cannot use solar energy at night and wind energy when the wind is not blowing. But we can store the generated electricity in some other forms and utilize it whenever required. That is how water splitting bridges the gap and has emerged as a very promising energy storage technology”, said Professor Masayuki Yagi who conducts research on energy storage materials and technology at the Department of Materials Science and Technology, Faculty of Engineering/Graduate School of Science and Technology, Niigata University. Water splitting is one of the promising energy storage solutions which would potentially drive the world towards a hydrogen fuelled economy.
The water dissociation process, alternatively known as artificial photosynthesis, traditionally employs electricity to split the water molecule through two half reactions in an electrochemical cell. The hydrogen evolution reaction occurs at the cathode where hydrogen fuel is generated and the water oxidation occurs at the anode where breathable oxygen is released. Although water is a simple molecule that is constituted by only three atoms, the process of dissociating it is quite intense and challenging.
The initial energy, known in scientific terms as the overpotential, plays a crucial role in influencing the progress of the reaction. For the materials explored so far, the initial energy required to trigger the hydrogen evolution at the cathode and oxygen evolution at the anode is so high that the process escalates the overall cost of the reaction, thereby, adversely affecting its commercial utilization. This is particularly a major concern at the anode because the oxygen evolution reaction involves the transfer of four electrons which demands a higher initial energy as compared to the reaction at the cathode.
Prof. Yagi’s research team at the Niigata University in association with research collaborators at the Yamagata University are investigating on the electrocatalytic water splitting and to address the key shortcomings. They have been successful in developing an efficient water dissociation process using nickel based nano-compounds as anodes which has been published in as a scientific article in Energy & Environmental Science on 20th May.
In this study, Prof. Yagi’s team have observed that the nickel sulphide nanowires based anode has supported the reduction of initial energy that is required for the oxygen evolution reaction. “We have fabricated the anode using a unique motif of nickel sulfide nanowires stuffed into carbon nitride scabbards. The carbon nitride scabbards prevent the core region of NiSx rods from transforming to their oxide, thereby protecting them from further degradation. On the surface of the nickel sulphide nanowires, a thin oxide film is formed due to the contact with the electrolyte solution, which facilitates the oxygen evolution reaction” explained Prof. Yagi.
The research team has observed with the aid of advanced microscopy techniques and electrochemical measurements that the fabricated anode aids in reducing the initial energy, which accelerates the four-electron transfer process in the oxygen evolution reaction. The research finding of Prof. Yagi’s team has immense potential in improving the long-term performance and stability of the electrochemical cell.
This research study is an important milestone towards improving the efficiency of the water splitting technology. Prof. Yagi said, “This result is a great breakthrough in the electrocatalytic water splitting system and could undoubtedly contribute to realize the de-carbonized human society in near future.”
###
References:
[1] Yuki Tanahashi et al., ACS Appl. Energy Mater. 2020, 3, 12172 – 12184.
[2] Z. N. Zahran et al., ACS Appl. Energy Mater. 2021, 4, 1410 – 1420.
Funder: This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP17H06439, JP18H02071, in Scientific Research on Innovative Areas ”Innovations for Light-Energy Conversion (I4 Q5 LEC)”.
See the article: Zaki N. Zahran, Eman A. Mohamed, Yuta Tsubonouchi, Manabu Ishizaki, Takanari Togashi, Masato Kurihara, Kenji Saito, Tatsuto Yuia and Masayuki Yagi, “Electrocatalytic water splitting with unprecedentedly low overpotentials by nickel sulfide nanowires stuffed into carbon nitride scabbards”; Energy & Environmental Science; https:/
Media Contact
Masayuki Yagi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.