Credit: by Yansong Feng, Zhi Li, Qiqing Li, Jun Yuan, Langping Tu, Lixin Ning and Hong Zhang
The great application prospect in biology, medicine, optogenetics, photovoltaics and sustainability has enabled lanthanide ions-doped upconversion nanoparticles to attract widespread attention which derives mainly from their superior anti-Stokes spectroscopic property. However, the relatively low upconversion efficiency remains a major bottleneck on their way of actual applications. Internal OH- impurity is known as one of the main detrimental factors affecting the upconversion efficiency of nanomaterials. Different from surface/ligand related emission quenching which can be effectively diminished by, e.g., core/shell structure, internal OH- is easy to be introduced during synthesis but difficult to be quantified and controlled.
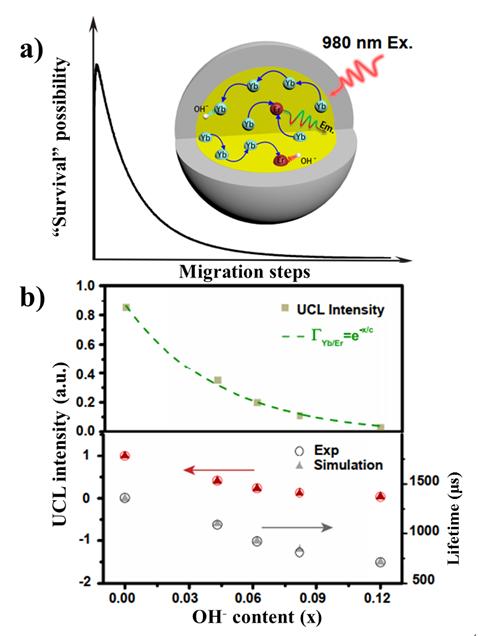
In a new paper published in Light Science & Application, a team of scientists, led by Professor Hong Zhang at van ‘t Hoff Institute for Molecular Sciences, University of Amsterdam and collaborators of Chinese Academy of Sciences and Anhui Normal University, have reported a progress in wide range quantitative adjustment of the internal OH- impurities in NaYF4:Yb/Er upconversion nanocrystals. From spectroscopy study and model simulation, they revealed the exponential relation between upconversion luminescence intensity and the quantity of internal OH- impurities, which is quantitatively attributed to the microscopic interactions between internal OH- and the sensitizers and activators, respectively. The internal OH- involved upconversion dynamical process is interpreted with a vivid concept of “Survivor effect”, i.e., the shorter the migration path of an excited state, the larger the possibility of its surviving from OH- induced quenching. The new insights will open new avenues for the construction of highly efficient upconversion materials.
By comparison with standard NaOH/D2O solution, the OH- content inside the upconversion nanoparticles could be quantified from Fourier-Transform Infrared spectroscopy (FTIR), where ultra-small nanoparticles were employed to promote the reliability of quantification. These scientists summarize the operation of their quantification approach:
“We utilized 0.1 M DCl (in D2O) instead of HCl (in H2O) to remove all the potential OH- in surface ligands/H2O molecules. Upconversion nanoparticles and DCl were mixed and stirred vigorously until the nanoparticles were completely transferred into the D2O phase. The nanoparticles were centrifuged and re-dispersed in pure D2O twice and finally dispersed in D2O and adjusted to 50 mg mL-1. The content of internal OH- were determined from its absorbance near 3400 cm-1, measured by FTIR.”
“The construction of Monte Carlo simulation model for the upconversion luminescence is another highlight of our work, which based on the hypotheses of three-dimensional random wandering of the excited states in the sublattice. Upconversion events resulting from the “collisions” of two or more excited states. The internal OH- effect was added in the simulation that led to higher non-radiative recombination rate of excited states and lower upconversion efficiencies, depending on the OH- content.” they added.
“The presented results clarify the role of OH- inside nanoparticles on the quenching of upconversion luminescence which enriches our comprehension towards upconversion mechanism. These results will promote the construction of highly efficient lanthanide -doped upconversion materials.” the scientists forecast.
###
Media Contact
Langping Tu
[email protected]
Related Journal Article
http://dx.




