Credit: ZHU Lei
Recently, Prof. WANG Junfeng from the High Magnetic Field Laboratory of the Hefei Institutes of Physical Science (HFIPS), together with international scholars, developed a novel circular permutated light-oxygen-voltage 2 (LOV2) to expand the repertoire of genetically encoded photoswitches, which will accelerate the design of novel optogenetic devices. The result was published in Nature Chemical Biology.
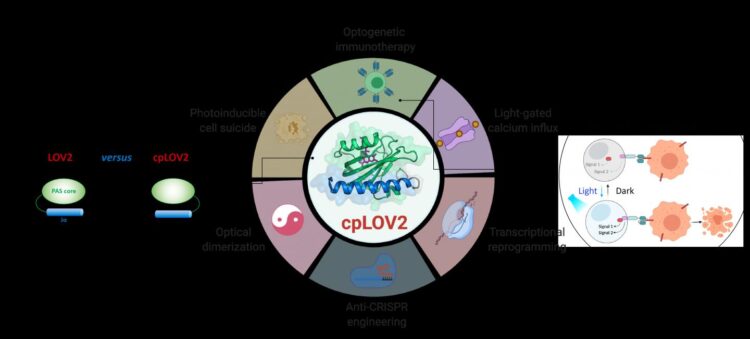
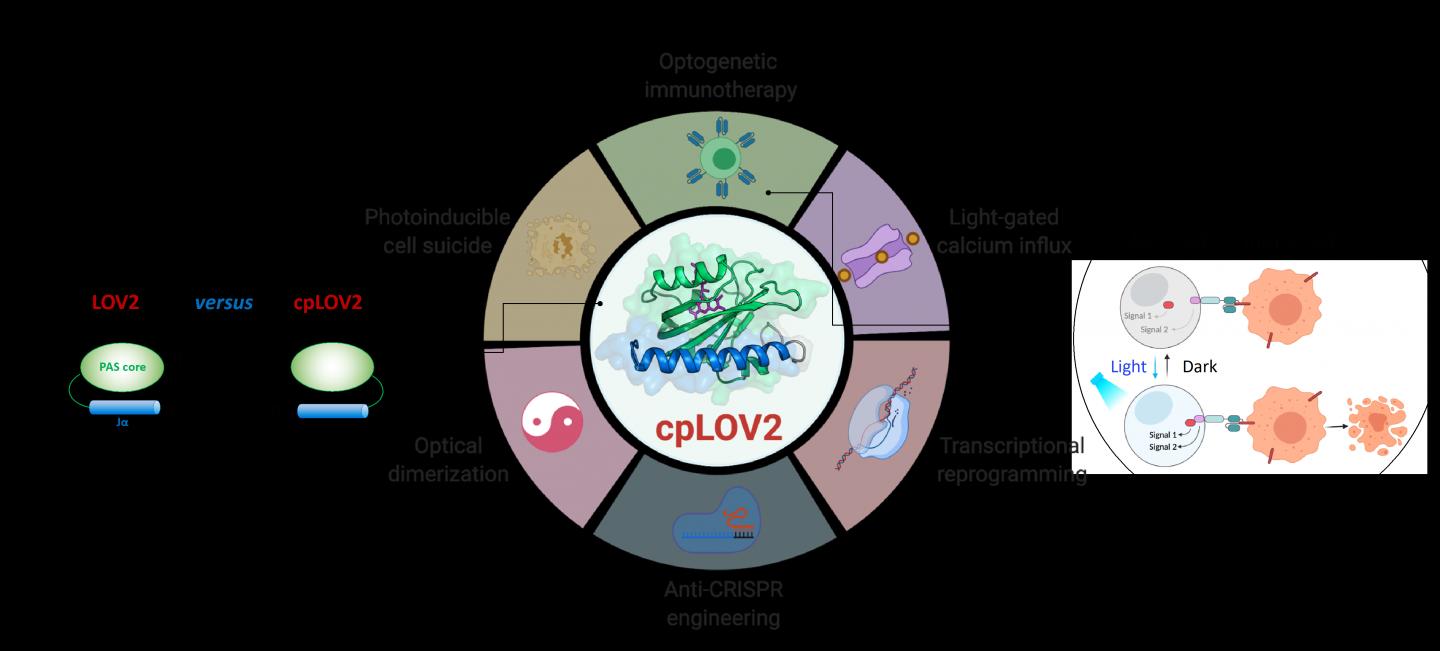
LOV2 domain is a blue light-sensitive photoswitch. In a typical LOV2-based optogenetic device, an effector domain is fused after the C-terminal Jα helix of LOV2, intending to cage the effector via steric hindrance in the dark. On photostimulation, light-triggered unfolding of the Jα helix exposes the effector domain to restore its function. Crafting a LOV2-based photoswitchable protein often takes tremendous engineering efforts to optimize each component and the connecting linker in between. Therefore, it is desirable to expand the current optogenetic toolbox by creating new modules that simplify these steps.
In this study, the researchers designed cpLOV2 using circular permutation, a robust protein engineering approach previously used to evolve new variants of genetically encoded fluorescent probes and biocatalysts. The nitrogen (N) and carbon (C) termini of cpLOV2 were created at the N-terminus of Jα helix while the old ones were connected by a glycine and serine-rich linker. Therefore, the effector could be fused before the N-terminal Jα of cpLOV2 in addition to the C-terminus in LOV2.
Using high resolution NMR spectroscopy and other techniques, the researchers demonstrated that the structural integrity and function of light-induced Jα dissociation of cpLOV2 are well maintained. cpLOV2 was also well worked in LOVTRAP and improved light-induced dimer (iLid), both are LOV2 based optical heterodimerization systems.
cpLOV2 provided more choices for optogenetic application developments. The researchers generated a series of hybrids by fusing LOV2 or cpLOV2 with different Ca2+ channel-activating and autoinhibition fragments derived from stromal interaction molecule 1, and found several novel cpLOV2 based optical actuators to gate ORAI1 Ca2+ channel, therefore they demonstrated that cpLOV2 could afford new caging surfaces to overcome limitations associated with wild type LOV2.
For effectors required a free N terminus to execute its full function, cpLOV2 is a better choice. A key protein involved in necroptosis, mixed lineage kinase domain-like (MLKL) protein, was the successful caged and uncaged in cpLOV2-MLKL but not MLKL-LOV2 to optical control of cell suicides.
Chimeric antigen receptor (CAR) T cell therapy has emerged as a promising immunotherapeutic approach. However, the uncontrollable CAR T cell activity during therapy would cause severe side-effects e.g. cytokine release syndrome in some patients. Researchers designed cpLOV2 based optical heterodimerization systems (cpLID), and constructed photo-tunable split CAR (optoCAR). The therapeutic optoCAR T cells can be specifically activated by CD19 tumor antigen and blue light, and then proliferate to kill CD19+ Raji lymphoma cells.
In mouse model implanted with CD19+ Raji cells, researchers used upconversion nanoparticles (UCNPs) to convert the high tissue penetrative near-infrared light to blue light and activate the injected optoCAR T cells and achieved highly effective therapy of lymphoma tumor. OptoCAR T cells developed in this study permit the spatiotemporal and reversible control of T cell activities and cytokine production.
These encouraging results suggest optoCAR T cells could mitigate potential side effects without losing therapeutic efficacy. In the future, they plan to try “optogenetic immunotherapy” to treat different types of cancer.
###
Media Contact
ZHAO Weiwei
[email protected]
Original Source
http://english.
Related Journal Article
http://dx.