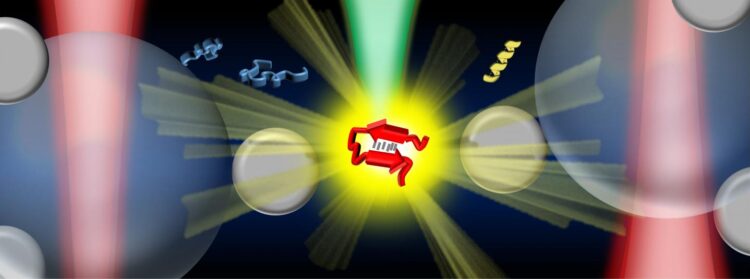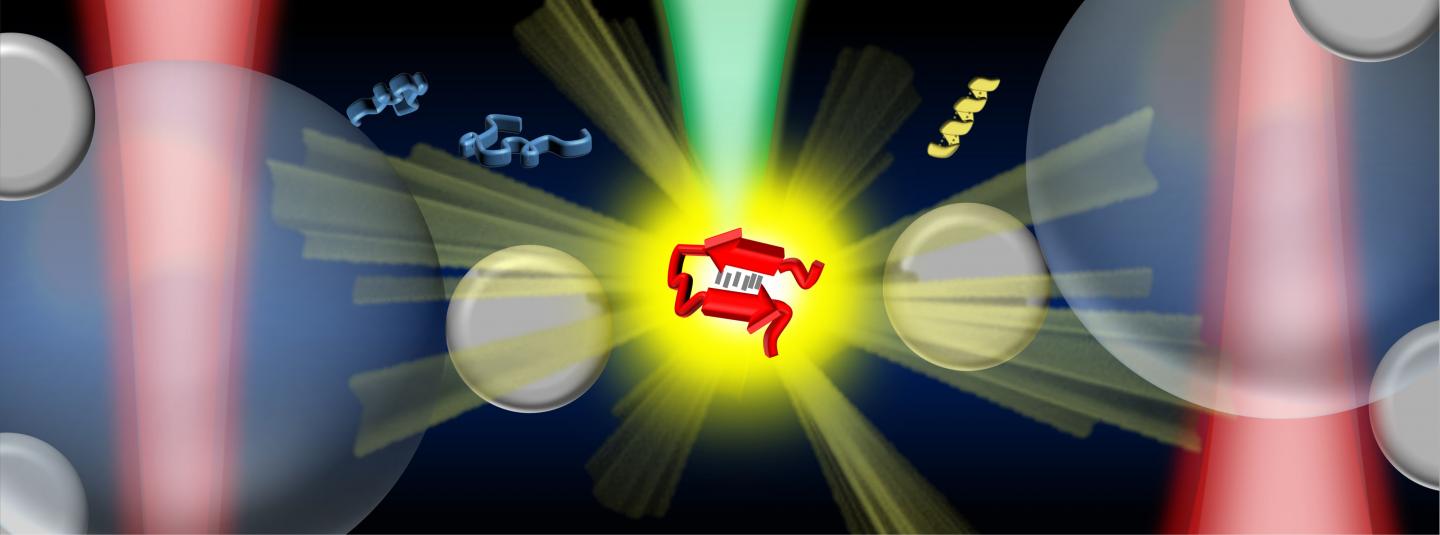
Credit: Vince St. Dollente Mesias, Jinqing Huang / The Hong Kong University of Science and Technology
It is challenging to analyze proteins at low concentrations, especially for those in a mixture of various conformations such as intrinsically disordered proteins (IDPs). A research team led by Prof. HUANG Jinqing, Assistant Professor of Department of Chemistry at The Hong Kong University of Science and Technology (HKUST), has developed optical tweezers-coupled Raman spectroscopy that can directly probe the structural features of alpha-synuclein, an IDP closely linked to Parkinson’s disease, at the physiological concentration by focusing on individual protein molecules.
IDPs play an important role in biological processes and many of them are associated with incurable neurodegenerative diseases. As a typical IDP, alpha-synuclein lacks a stable 3-D architecture known as secondary structures. It spontaneously undergoes conversions from one secondary structure to another, which could eventually result in the buildup of protein aggregates involved in Parkinson’s disease pathology. However, the transient species during the conversion possess various structures and exist in low population among a dynamic equilibrium mixture. Therefore, their structural features are usually buried under the detection results from traditional measurement techniques, which average the signals detected from large sample quantities and long detection time.
In the study, Prof. Huang and her collaborators integrate optical tweezers and surface-enhanced Raman spectroscopy (SERS) in a novel platform to generate tunable and reproducible SERS enhancements with single-molecule level sensitivity in aqueous environments, in order to characterize these IDPs while maintaining their intrinsic heterogeneity with great biological significance. Specifically, a hotspot can be visualized and controlled by optical tweezers to allow proteins to go through in a microfluidic flow chamber, which makes it convenient to adjust the measurement parameters in the real time for the in situ spectroscopic characterizations. It directly identifies the structural features of the transient species of alpha-synuclein among its predominant monomers at physiological concentration of 1?μM by reducing the ensemble averaging in quantity and in time, providing profound insight to understand the initiation of amyloid protein aggregation. Hence, this SERS platform has great potential to reveal the structural information of IDPs in the dynamic, heterogeneous, and complex biological systems.
“Our strategy enables the precise control of the hotspot between two trapped micrometer-size silver nanoparticle-coated silica beads to improve the SERS efficiency and reproducibility in aqueous detections. Except for the tunable SERS enhancement, the integrated optical tweezers also offer sub-nanometer spatial resolution and sub-piconewton force sensitivity to monitor light-matter interactions in the plasmonic hotspot for extra physical insight. More importantly, our method opens a new door to characterize the transient species of IDPs in dilute solutions, which remains a significant challenge in the biophysics community. Ultimately, it will be exciting to fully exploit the precise force manipulation of the integrated optical tweezers to unfold a single protein inside the controllable hotspot and resolve its structural dynamics from the endogenous molecular vibrations by the integrated Raman spectroscopy.” said, Prof. Huang.
The study was recently published in the scientific journal Nature Communications.
###
Media Contact
Sam Li
[email protected]
Related Journal Article
http://dx.