Credit: Graphic: Steffen Wolf
Consider for a moment a tree swaying in the wind. How long does it take for the movement of a twig to reach the trunk of the tree? How is this motion actually transmitted through the tree? Researchers at the University of Freiburg are transferring this kind of question to the analysis of proteins – which are the molecular machinery of cells. A team of researchers lead by Prof. Dr. Thorsten Hugel of the Institute of Physical Chemistry, and Dr. Steffen Wolf and Prof. Dr. Gerhard Stock of the Institute of Physics are investigating how the signals that cause structural changes in proteins travel from one site to another. They are also trying to determine how fast these mechanisms take place. Until now, researchers have been unable to analyze the precise rate of signal transfer because it involves many time scales – ranging from nanoseconds to seconds. The researchers in Freiburg, however, have now achieved such resolution by combining various experiments, simulations, and theoretical studies. They are publishing their results in the scientific journal Chemical Science.
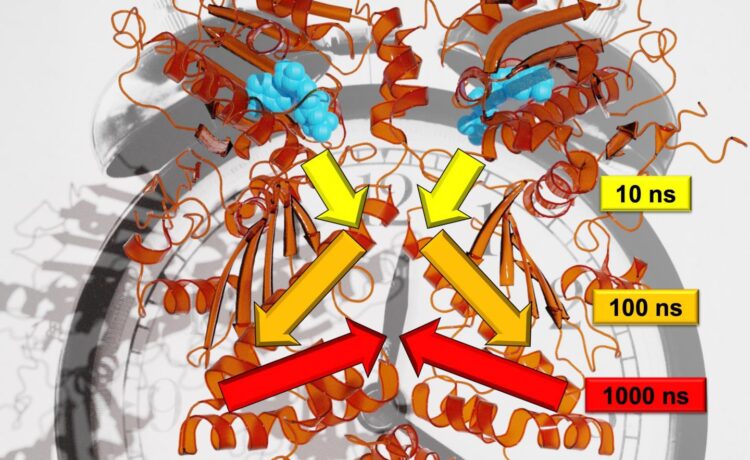
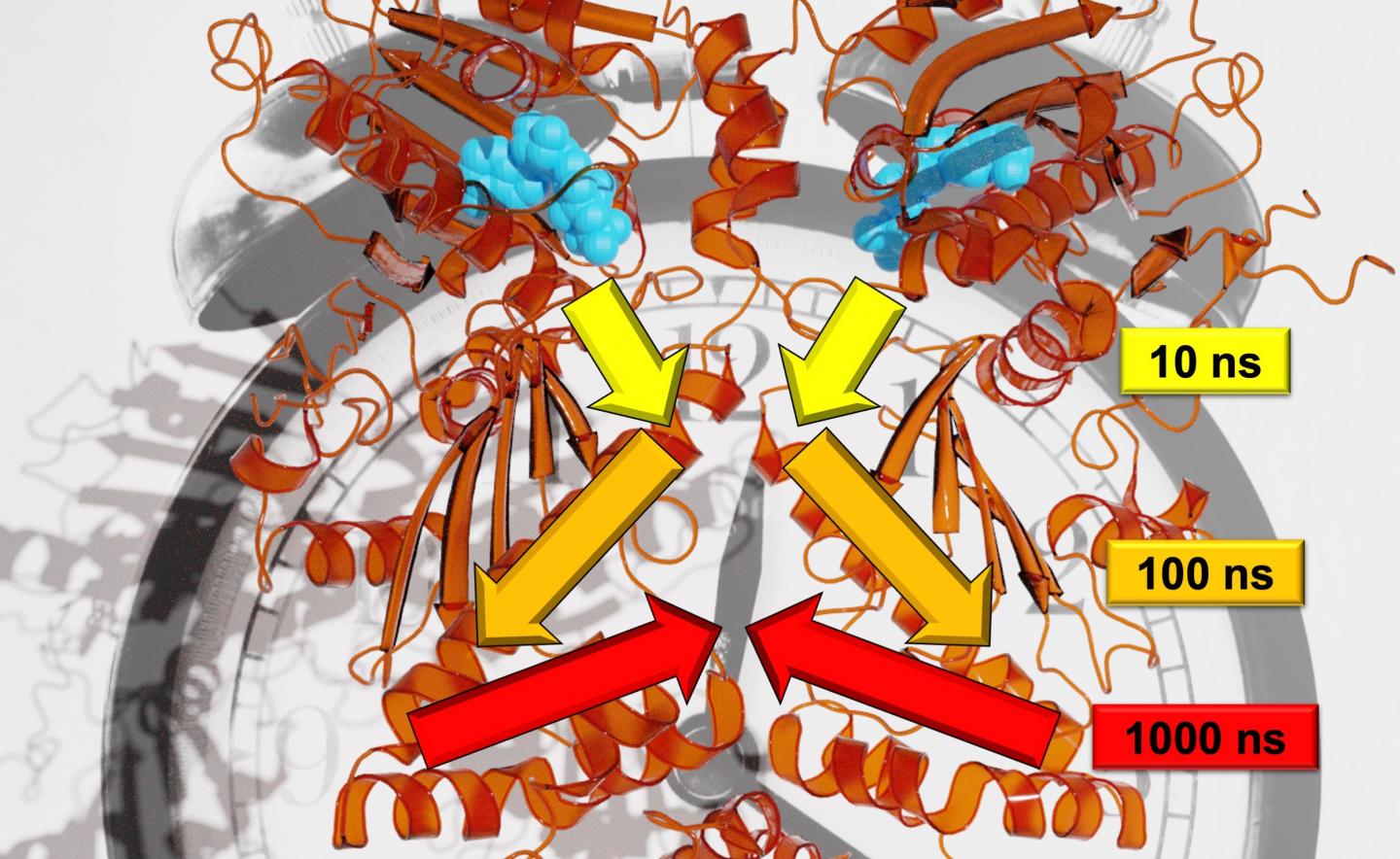
In contrast to trees, the movements for the protein analyzed in the study, Hsp90, unfold on logarithmic time scales. Every large movement takes around ten times as long as the small, individual movements that make up the larger one. Wolf explains, “For example, a twig moves on a time scale of seconds; the branch with ten seconds; and the trunk with 100 seconds.” Using a combination of state-of-the-art experimental and theoretical methods enabled the researchers to monitor allosteric communication, in other words, to show how a reaction process in Hsp90 altered a remote protein binding site. According to Stock, the team discovered the hierarchy of dynamics that this allosteric process unfolds on, which include the nanosecond to millisecond timescales and length scales from picometers to several nanometers.
What is more, the reaction process in Hsp90 is coupled with a structural change in the single amino acid Arg380. Arg380 then transmits structural information to a subdomain of the protein, and ultimately, passes it on to the protein as whole. The resulting change in structure closes a central binding site of the protein, thereby enabling it to fulfill new functions. The University of Freiburg researchers suspect that similar hierarchical mechanisms such as the one demonstrated in the Hsp90 protein are also of fundamental importance in signal transfer within other proteins. Hugel says that this could be useful for using drugs to control proteins.
###
The researchers who worked on the study are members of the Excellence Cluster – Centre for Integrative Biological Signalling Studies (CIBSS), the Collaborative Research Center 1381 “Dynamic Organization of Cellular Protein Machineries,” and the Research Unit 5099 “Reducing complexity of nonequilibrium systems,” which is supported by the German Research Foundation, DFG.
Original publication:
Wolf, S., Sohmen, B., Hellenkamp, B., Thurn, J., Stock, G., Hugel, T. (2021): Hierarchical dynamics in allostery following ATP hydrolysis monitored by single molecule FRET measurements and MD simulations. In: Chemical Science. DOI: 10.1039/D0SC06134D
Contact:
Dr. Steffen Wolf and Prof. Dr. Gerhard Stock
Institute of Physics
Faculty of Mathematics and Physics
University of Freiburg
Tel.: 0761/203-5913
E-mail: [email protected]; [email protected]
Prof. Dr. Thorsten Hugel
Institute of Physical Chemistry
Faculty of Chemistry and Pharmacy
University of Freiburg
Tel.: 0761/203-6192
E-Mail: [email protected]
Media Contact
Prof. Dr. Thorsten Hugel
[email protected]
Related Journal Article
http://dx.