Understanding intermediates of the electrochemical CO2 reduction to ethylene. Encouraging catalyst design-a key component of CO2 utilization using renewable energy.
Credit: Korea Institue of Science and Technology(KIST)
In recent times, “electrochemical conversion (e-chemical)” technology-which converts carbon dioxide to high-value-added compounds using renewable electricity-has gained research attention as a carbon capture utilization (CCU) technology. This green carbon resource technology employs electrochemical reactions using carbon dioxide and water as the only feedstock chemical to synthesize various compounds, instead of conventional fossil fuels. Electrochemical CO2 conversion can produce value-added important molecules in a petrochemical industry such as carbon monoxide and ethylene. Ethylene, referred to as the “rice of the industry”, is widely used to produce various chemical products and polymers, but it is more challenging to produce from electrochemical CO2 reduction. The lack of understanding of the reaction pathway by which carbon dioxide is converted to ethylene has limited to develop high-performance catalyst systems and to advance its application to produce more valuable chemicals.
To overcome this limitation, a domestic research team in South Korea has made a breakthrough in unveiling a key path-triggering intermediate in the ethylene production reaction. Dr. Yun-Jeong Hwang and her team at the Clean Energy Research Center of the Korea Institute of Science and Technology (KIST) has announced that they have successfully observed the key intermediates adsorbed on the surface of a copper-based catalyst during electrochemical CO2 reduction to ethylene production and analyzed its behavior in real time. This was research was conducted in collaboration with Professor Woo-Yul Kim and his team at the Department of Chemical and Biological Engineering, Sookmyung Women’s University (President: Yoon-Geum Jang), with the support of the climate change response technology development project (Next Generation Carbon Upcycling Project Group, led by Ki-Won Jun).
It has been reported that copper-based catalysts can promote carbon dioxide conversion to synthesize not only relatively simple carbon monoxide or formic acid but also multi-carbon compounds such as ethylene and ethanol. Nevertheless, the development of control technology for selectively synthesizing high-value-added compounds has been limited because of the absence of information on major intermediates and pathways of the carbon-carbon bond forming reaction.
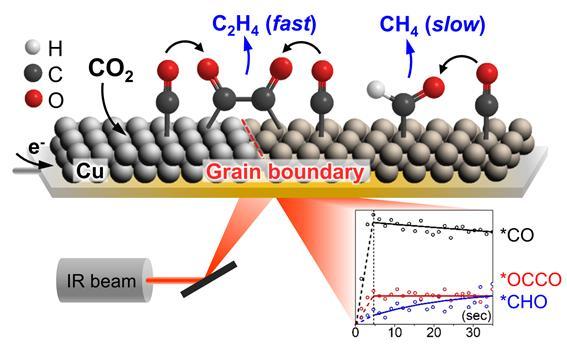
Through infrared spectroscopy, the research team observed the intermediate responsible for the formation of the ethylene intermediate (*OCCO) as well as the one responsible for the production of methane (*CHO). The intermediate is a dimer of carbon monoxide formed during the carbon dioxide conversion reaction on the surface of the copper nanoparticle catalyst. As a result, carbon monoxide and the ethylene intermediate (*OCCO) were produced at the same time, whereas the methanol intermediate (*CHO) was produced relatively slower than the two other intermediates, suggesting the possibility of further improving the selectivity of compound formation on the catalyst surface by controlling the reaction pathway.
In addition, copper hydroxide (Cu(OH)2) nanowire was proposed as a promising catalyst that exhibits excellent performance toward ethylene production by accelerating carbon-carbon bond formation. The research team found that there were multiple catalytic sites on which carbon monoxide can be adsorbed on the surface of the catalyst derived from copper hydroxide and that carbon monoxide adsorbed on a specific site quickly forms an intermediate through carbon-carbon bond formation. Further research on this intermediate is expected to contribute significantly to the identification of the active sites for the carbon-carbon bond forming reaction, which has been a subject of debate.
“The success of this study is significant in that it has presented a key direction for basic research related to artificial photosynthesis that has been unexplored in Korea through a joint investigation by the research institute as well as the university,” said Dr. Yun-Jeong Hwang of KIST. “Based on this, we will be able to contribute significantly to the growth of next-generation carbon resource conversion technology based on sustainable energy in response to climate change.”
###
This study was conducted with a grant from the Ministry of Science and ICT (MSIT), as part of the Institutional R&D Program of KIST and the Climate Change Technology Development Program (Next-Generation Carbon-as-a-Resource Project Group, Director Gi-won Jeon). The findings were reported in the latest edition of the international journal, Energy & Environmental Science (IF: 30.289, Top 0.189% in the field of JCR).
Media Contact
Do-Hyun Kim
[email protected]
Related Journal Article
http://dx.




