Elucidation of the mechanism mediating the autophagosome membrane expansion
Credit: Nobuo N. Noda & Kazuaki Matoba
Drs. Nobuo Noda (Director) and Kazuaki Matoba (Senior Researcher) et al. at the Institute of Microbial Chemistry (BIKAKEN, Tokyo, Japan) discovered that Atg9, one of the proteins that function to mediate autophagy, has phospholipid-translocation activity (the lipid scramblase activity (1)) between the two layers of the lipid bilayer (2) and elucidated that the protein’s activity brings about autophagosome (3) membrane expansion.
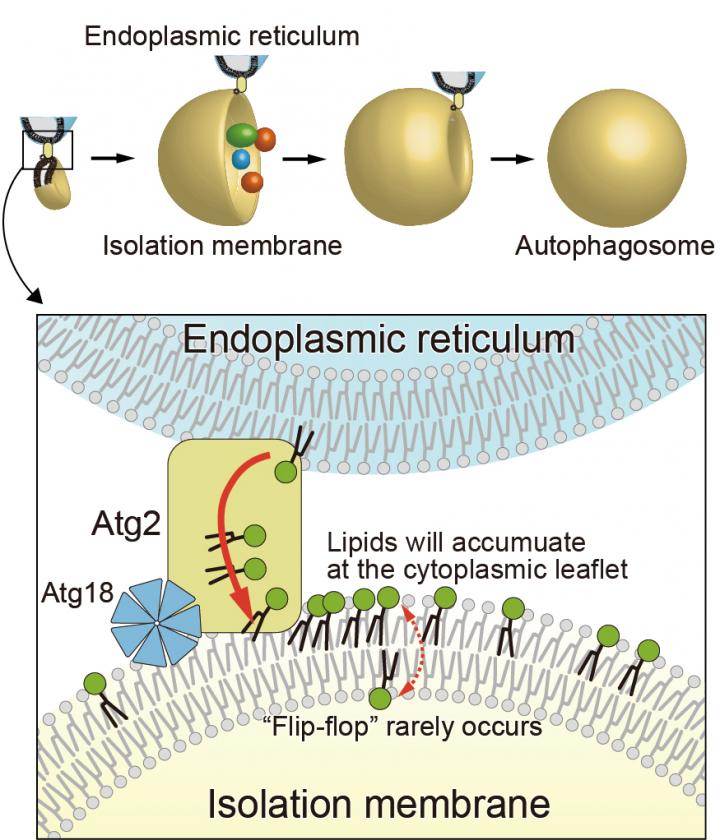
Autophagosome formation is an essential step in determining the target of degradation in autophagy, which is one of the mechanisms of intracellular protein degradation. Although this research group has previously revealed that Atg2, a lipid transfer protein, transfers phospholipids from the endoplasmic reticulum, the manner in which the membrane is expanded using the transported phospholipids remains unknown.
The research group demonstrated that yeast and human Atg9, a membrane protein of unknown function, exhibits lipid scramblase activity through in vitro experiments. Moreover, as a result of examining the three-dimensional structure of yeast Atg9 by cryo-electron microscopy (4), they found that Atg9 has pores connecting the two membrane leaflets of the lipid bilayer. They also found that the mutations to the pore-forming amino acids resulted in losing the lipid scramblase activity of Atg9 in vitro and inhibited the autophagosome formation in yeast. Consequently, they revealed a brand-new mechanism that Atg9 is a novel scramblase (1); in conjunction with Atg2, a lipid transfer protein, these two proteins act to form autophagosomes.
The elucidation of the mechanisms of isolation membrane expansion, which have been a long-standing issue in the field of autophagy holds promise in accelerating research that will contribute to a complete understanding of the molecular mechanisms of autophagosome formation. It is also expected to promote the research and development of treating and preventing various diseases through the artificial control of autophagy due to deepening our understanding of autophagosome formation mechanisms.
###
The present research collaborated with the groups of Drs. Masahide Kikkawa (Professor, The University of Tokyo), Hitoshi Nakatogawa (Associate Professor, Tokyo Institute of Technology), Yoshinori Ohsumi (Honorary Professor), Toyoshi Fujimoto (Research Professor, Juntendo University), Yuji Sugita (Principal Investigator, RIKEN), and So Iwata (Professor, Kyoto University) in JST Strategic Basic Research Programs (CREST).
(1) The lipid scramblase activity, Scramblase
Although the phospholipids generally cannot translocate freely between the lipid bilayers, the activity that promotes the translocation is referred to as the lipid scramblase activity. The protein with this activity is referred to as scramblase.
(2) The phospholipids and lipid bilayer
The phospholipids, a generic term for lipids with phosphate esters, have hydrophilic and hydrophobic parts. They are not soluble in water by themselves because of the hydrophobic part. Still, they can exist stably in an aqueous solution by the lipid bilayer formation facing each other’s hydrophobic part. The various lipid membranes of the cell are composed of a lipid bilayer consisting mainly of phospholipids.
(3) Autophagosome
It is a double-membrane organelle newly produced in the cytoplasm due to the induction of autophagy. Everything enclosed by the autophagosome (such as various proteins and nucleic acids) is transported to the lysosome (yeast vacuoles). It is degraded by a function of a group of degrading enzymes within the lysosome.
(4) Cryo-electron microscopy
It is a type of transmission electron microscopy measuring a sample at a low temperature. In recent years, it has exhibited remarkable progress and is widely used as a method for determining the three-dimensional structure of proteins with high resolution.
Media Contact
Nobuo Noda
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




