Credit: ©Science China Press
Hydrogels are a class of soft materials resembling native soft tissues, which consist of cross-linked 3D networks formed by covalent bonds or physical interactions. Due to the high water content and viscoelastic nature, hydrogels are well suitable to encapsulate cells and bioactive factors, and therefore extensively investigated for applications in drug delivery and tissue engineering.
The condensation reactions between carbonyl groups (aldehyde, ketone) and N-nucleophiles (primary amine, hydrazide, and aminooxy) undergo spontaneously in physiological conditions, yielding Schiff base, hydrazone, and oxime, respectively, and have been widely used for the construction of hydrogels. The Schiff base is prone to hydrolysis, while the formation of hydrazone and oxime linkages, much more stable than Schiff base, proceeds at second-order rate constants below 0.1 M-1 s-1 at neutral condition. Consequently, the hydrogels based on the carbonyl/N-nucleophile reaction are commonly prepared at relatively high concentration of cross-linking moieties or in the presence of catalysts, giving rise to toxicity issues. Thus, fast and catalyst-free cross-linking strategy is of great significance for the construction of hydrogels for biomedical applications.
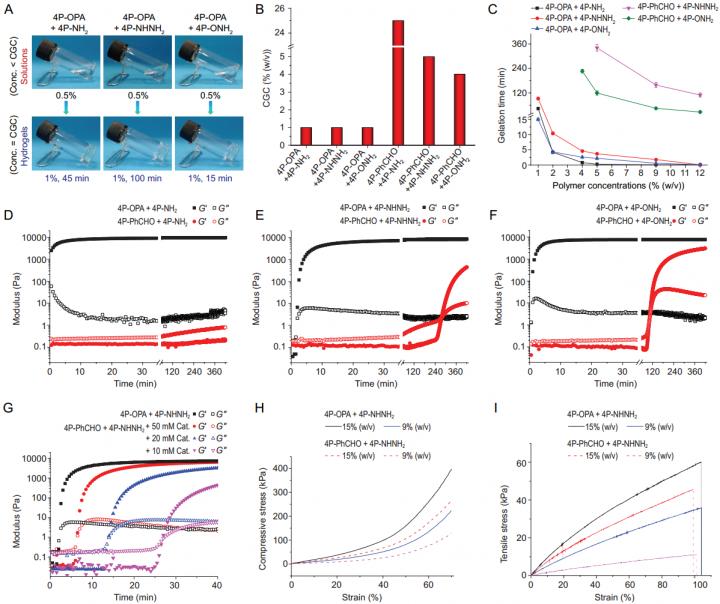
Recently, Prof. Xuesi Chen and colleagues at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, proposed a new crosslinking strategy based on the condensation reaction between o-phthalaldehyde (OPA) and N-nucleophiles for hydrogel formation (Figure 1). When four-arm poly(ethylene glycol) (4aPEG) end-capped with OPA was mixed with various N-nucleophile-terminated 4aPEG as building blocks, hydrogels were formed with superfast gelation rate, enhanced mechanical strength, and markedly reduced critical gelation concentrations (CGCs) as low as 1% (w/v). The CGCs are merely 1/25~1/4 of those for the benzaldehyde-based counterparts, which diminishes the toxicity issue of high aldehyde content. No hydrogel formation (Schiff base) or considerable long gelation time (27~100 min for oxime, acylhydrazone) was observed for the benzaldehyde-based mixtures at 12% (w/v), while free-standing hydrogels could form within few seconds for the OPA-based hydrogels at the same polymer concentration. Besides, the mechanical properties of the OPA-based hydrogels were markedly strengthened (Figure 2).
Small molecule model reactions indicate the key to these cross-links is the rapid formation of heterocycle phthalimidine product or isoindole (bis)hemiaminal intermediates, depending on the N-nucleophiles. The second-order rate constant for the formation of phthalimidine between OPA and methylamine (4.3 M-1 s-1) is over 3000 times and 200 times higher than those reported for acylhydrazone and oxime formation from benzaldehyde in phosphate buffer, respectively, and comparable to many cycloaddition click reactions.
Due to the high efficiency and versatility of OPA/N-nucleophile chemistry, various hydrogels can be readily prepared from naturally derived polysaccharide, proteins, and synthetic polymers with no need of tedious chemical modification by using OPA-terminated 4aPEG as cross-linker. The versatile building blocks provide the hydrogels with tunable biodegradability and bioactivity.
Moreover, biofunctionality is easily introduced to the hydrogels by incorporating amine-bearing peptides via the one-step reaction between OPA and amino group during the crosslinking process. For instance, the c(RGDfK) incorporated hydrogels well supported the adhesion and proliferation of fibroblasts on the hydrogel surface. Taken together, this work provide a universal strategy for the rapid construction of mechanical strengthened and biofunctional hydrogels.
###
See the article:
A fast and versatile cross-linking strategy via o-phthalaldehyde condensation for mechanically strengthened and functional hydrogels
National Science Review, nwaa128,
https:/
Media Contact
BEI YAN
[email protected]
Related Journal Article
http://dx.




