Credit: ©Science China Press
As the most abundant constituent in Earth’s atmosphere, dinitrogen (N2) is the main nitrogen source of N-containing compounds in the Earth. Therefore, N2 fixation and activation are essential both for nature and humans. Nevertheless, the high bond dissociation energy (942 kJ/mol) and large HOMO-LUMO gap (10.82 eV) make N2 exhibit extremely low reactivity and be regarded as an inert gas.
Currently, the N2 activation and conversion in nature and industry mainly rely on two pathways, in which ammonia (NH3) is the product. In nature, nitrogenase metalloenzymes transfer N2 into NH3 at ambient temperature and pressure. In industry, more than 170 million metric tons of NH3 is produced from the Haber-Bosch process annually, in which N2 reacts with dihydrogen (H2) under harsh condition in the presence of metal catalysts. This NH3 synthesis process consumes about 1-2% of the world’s annual energy supply along with the huge CO2 emission.
Compared to NH3-based N2 fixation process, an alternative route of N2 fixation is the direct conversion of N2 into N-containing organic compounds under mild condition. This approach is always targeted because it provides the potential solution to develop a sustainable system with reduced fossil-fuel requirements.
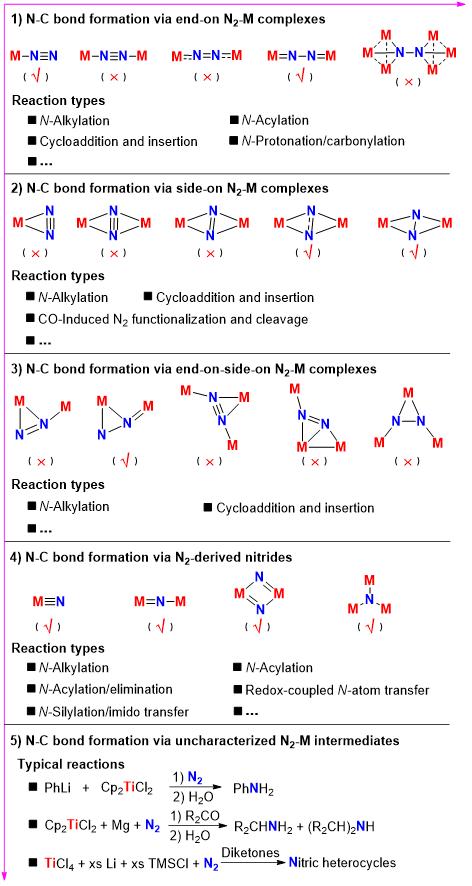
In a new review published in the National Science Review, Zhenfeng Xi et al. summarize the previous works of transition-metal mediated direct conversion of N2 into organic compounds via N-C bond formation at metal dinitrogen complexes. The review is organized by the coordination modes of the complexes (end-on, side-on, end-on-side-on, etc.) that are involved in the N-C bond formation steps, and each part is arranged in terms of reaction types (N-alkylation, N-acylation, cycloaddition, insertion, etc.) between metal dinitrogen complexes and carbon-based substrates. Besides, earlier works on one-pot synthesis of organic compounds from N2 via ill-defined intermediates are also briefed by the authors.
Besides the homogeneous stoichiometric thermochemical reaction systems, the sporadically reported syntheses involving photochemical, electrochemical, heterogeneous thermo-catalytic reactions are also discussed in this overview.
In the review, the authors point out that some synthetic cycles about direct conversion of N2 into organic compounds have also been developed in recent decades. However, all of these reactions are stoichiometric and the catalytic system for the direct introduction of N2 into organic compounds has not been realized yet. The main factors that prevent these complete synthetic cycles from becoming catalytic process are the rigorous reaction conditions of the N-C bond formation and N-containing organic compounds releasing steps in these cycles, which are incompatible with the preparation steps for metal dinitrogen complexes.
To provide readers with perspectives of future research particularly in direct catalytic and efficient conversion of N2 into N-containing organic compounds under mild conditions, the authors likewise outline the potential development directions. They forecast that the research topics of “new reaction types and systems for N-C bond formation”, “polynuclear metal species cooperative N2 scission and functionalization”, “main group elements promoted N-C bond formation”, “photochemistry and electrochemistry involved N-C bond formation”, “heterogeneous catalysis systems for conversion of N2 into organic compounds” would get more attention in the future.
###
See the article:
Ze-Jie Lv, Junnian Wei, Wen-Xiong Zhang, Ping Chen, Dehui Deng, Zhang-Jie Shi, and Zhenfeng Xi
Direct transformation of dinitrogen: synthesis of N-containing organic compounds via N-C bond formation
Natl Sci Rev (2020)
https:/
Media Contact
Zhenfeng Xi
[email protected]
Related Journal Article
http://dx.




