Credit: ©Science China Press
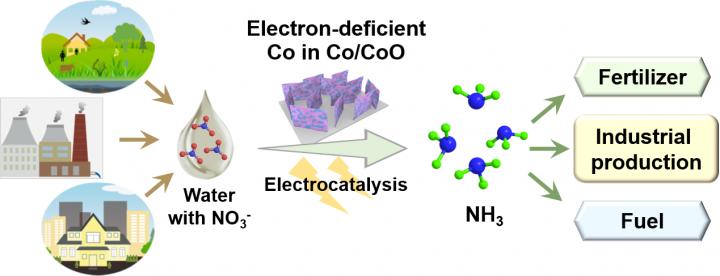
Ammonia has been widely used for agricultural fertilizers and industrial productions. Additionally, NH3 is expected to serve as next-generation green energy carriers due to its high energy density, low liquefying pressure, small air-fuel ratio and no carbon dioxide emission. At present, NH3 is mainly manufactured through the conventional Haber-Bosch process, which is energy-intensive and releases ~1.5% of global CO2 into the atmosphere. During the past years, electrocatalysis and photo(electro)catalysis of nitrogen gas and water into NH3 at ambient conditions have attracted great attention, but the Faradaic efficiency is greatly hampered by the high dissociation energy of N?N bonds (941 kJ mol-1) and the competitive reaction of H2 evolution. Thus, developing a new route for the ammonia synthesis under mild conditions is urgently desired.
As we all known, excessive nitrates (NO3-) exist in surface and underground water due to the overuse of nitrogen-based fertilizers and the discharge of industrial and domestic sewages, threating the human health. Considering that the dissociation energy of N-O bonds in nitrates is only 204 kJ mol-1 and ammonia can be easily reclaimed from its aqueous solution, it is of great interest to use nitrate contaminants as nitrogen source and water as hydrogen source for the electrochemical synthesis of high value-added ammonia. However, the competitive reaction of H2 generation and the complex eight-electron reduction process retard the FE and selectivity of ammonia during electrocatalytic nitrate reduction reactions. Thus, elaborate design and construction of efficient electrocatalysts is critical.
Very recently, Yu’s research group in Tianjin University fabricated Co/CoO nanosheet arrays (Co/CoO NSAs), in which electron-deficient Co was constructed by the rectification effect of the Schottky contact between the metallic Co and semiconducting CoO. The heterostructured Co/CoO NSAs with electron-deficient Co exhibited excellent performances for the electrochemical reduction of nitrates to ammonia: 93.8% of Faraday efficiency and 91.2% of selectivity, which were much higher than that of the Co NSAs. 15N isotope labeling experiments proved that the produced ammonia originating from the nitrate electroreduction and the product was quantified with 1H NMR spectra. In-situ electrochemical tests were conducted to capture the intermediates and speculate the reaction path. Theoretical calculations revealed that the electrons transferred from Co to CoO at the Co/CoO interface, thus leading to the electron-deficient Co, can effectively inhibit both the competitive reaction of hydrogen evolution and the formation of by-products in the reduction process, thereby improving the Faraday efficiency and selectivity. This work offers a facile strategy to construct efficient electrocatalysts for ammonia synthesis from nitrate reduction powered by renewable electricity.
###
See the article: Yu Y, Wang C, Yu Y, Wang Y, Zhang B. Promoting Selective Electroreduction of Nitrates to Ammonia over Electron-Deficient Co Modulated by Schottky Rectifying Contact. Sci. China Chem., 2020, DOI: 10.1007/s11426-020-9795-x
http://engine.
Media Contact
Yifu Yu
[email protected]
Related Journal Article
http://dx.




