An international team has developed a sophisticated experimental technique at BESSY II to observe the formation of a metallic conduction band in electrolytes.
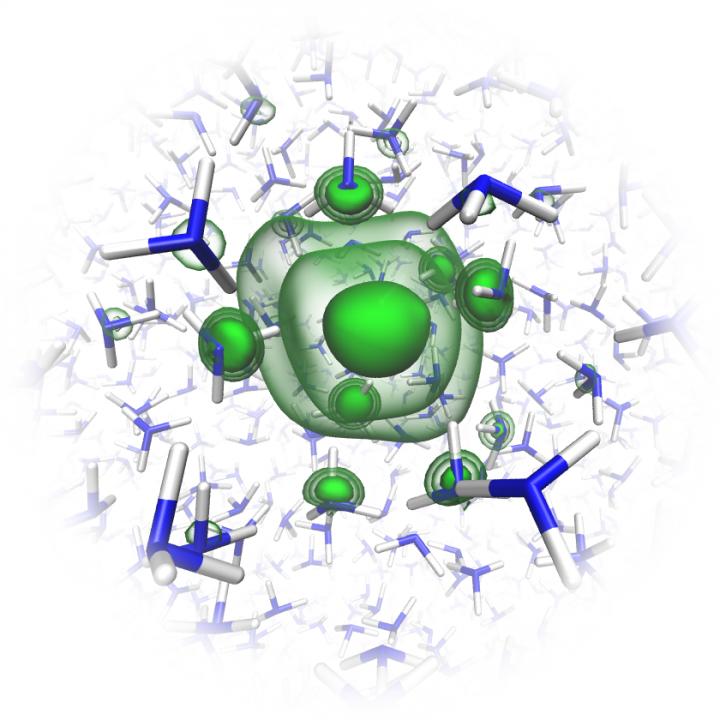
Credit: Charles Universität Prag/O. Maršálek & T. Martinek
The team then examined these liquid jets using soft X-rays at BESSY II and subsequently has been able to analyse this process in detail from the data they acquired combined with theoretical predictions. The work has been published in Science and appears even on the cover.
What distinguishes metals from other materials is generally well understood. In a metal, some of the atoms’ outer electrons move through the crystalline lattice in what is called a conduction band. This is how metals conduct electric current. In contrast to metals, the ions in electrolytes are disordered and electrical conductivity even decreases with increasing ion concentration. So how does metallic behaviour arise from the many individual metal atoms dissolved in the electrolyte? At what concentration and exactly how does a conduction band form, and how do the electron orbitals behave during this process?
A large international collaboration has now developed a sophisticated experimental technique that makes it possible to observe these processes for the first time. 17 authors at renowned institutes in Kyoto, Los Angeles, Paris, Prague and Berlin have contributed their expertise.
One of the main authors is Dr. Bernd Winter from the Fritz-Haber-Institut Berlin, who set up the experiment at BESSY II together with Dr. Robert Seidel, head of the HZB Operando Interfacial Photochemistry Young Investigator Group and his team. As a first step, the physicists dissolved alkali metals such as lithium and sodium in ammonia, forming solutions. The metal atoms become positively charged ions and their outer electrons are drawn into the liquid ammonia solution. These solutions are slightly blue at low metal concentrations, but as the metal concentration is increased, the blue colour becomes more intense until it transitions to a golden hue. This surprising color change is related to the electron states in the dissolved metals, the scientists assumed.
Using the SOL³PES instrument at the BESSY II U49/2-PGM-1 beamline that Seidel supervises, the team was able to study different concentrations of the alkali-metal/ammonia solutions as extremely narrow liquid jets under ultra-high vacuum using photoelectron spectroscopy. The solutions had to be cooled to about -60 degrees Celsius. At this temperature, ammonia is a liquid and its evaporation is sufficiently low. This enabled them to actually measure the transition from electrolyte to metal precisely.
“We were able for the first time to capture the photoelectron signal of the excess electrons in liquid ammonia. We observed a narrow peak at about 2 electron volts (eV), which indicates the presence of dissolved electrons and dielectrons”, says Winter. Seidel adds: “This also explains why the solution is initially blue at low and medium concentrations of metal ions: the solution absorbs light in the red region, which corresponds to the peak at 2 eV.” As a result, the solution appears slightly blue as long as there are only individual dissolved electrons. This blue colour intensifies with the appearance of the first “electron pairs”- called dielectrons. The colour changes to golden as the alkali metal concentration increases. At the same time, this narrow absorption peak widens into a band with a sharp Fermi edge in the spectrum, as is characteristic of metals, accompanied as well by signals associated with collective excitations (plasmons) – characteristic of free metallic electrons.
“The groups headed by the theoreticians Pavel Jungwirth and Ondrej Marsalek in Prague had been able to model the electronic structure of solvated electrons in solution in advance”, says Winter. “We found that the binding energies they calculated fit very well with our experimentally determined values. This gave us confidence in our interpretation of the X-ray data.”
The work is being published in Science because it makes an important contribution to the fundamental understanding of the transition from a non-conducting to metallic character in electrolytes. Moreover, there are even practical applications of solvated electrons, i.e electrons in solution, in organic chemistry as reducing agents for aromatic systems, in battery eletrolytes, and electronic capacitors.
###
Science (2020): Photoelectron spectra of alkali metal?ammonia microjets: From blue electrolyte to bronze metal
Tillmann Buttersack, Philip E. Mason, Ryan S. McMullen, H. Christian Schewe, Tomas Martinek, Krystof Brezina, Martin Crhan, Axel Gomez, Dennis Hein, Garlef Wartner, Robert Seidel, Hebatallah Ali, Stephan Thürmer, Ondrej Marsalek, Bernd
Winter, Stephen E. Bradforth, and Pavel Jungwirth
Media Contact
Robert Seidel
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




