Evasins offer promising pathway for anti-inflammatory medicine
Credit: University of Sydney
Anti-inflammatory proteins in tick saliva have been synthetically produced for the first time, a promising step towards new therapeutic treatments.
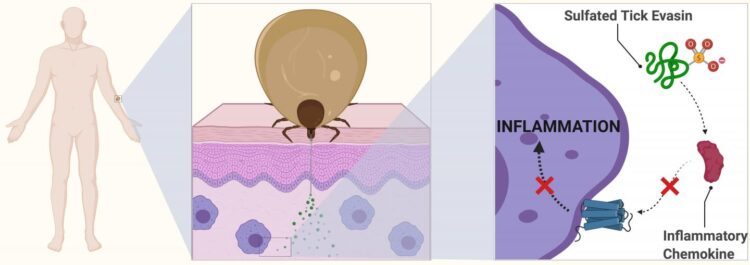
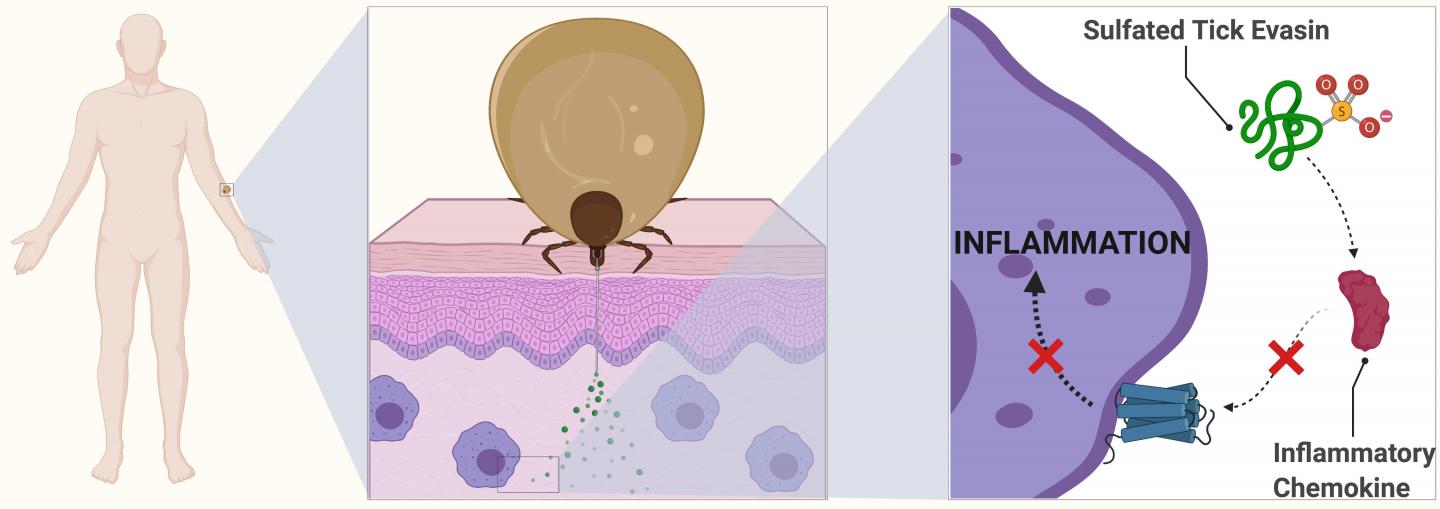
Evasins, as the proteins are known, act in human blood to suppress a class of transmitter proteins, which is why when bitten, we often don’t notice a tick has burrowed into our skin. Scientists now want to see how these proteins can be used for treating human diseases, including potential application for lung inflammation in respiratory illness, such as COVID-19.
Their research is published today in PNAS, the Proceedings of the National Academy of Sciences of the United States of America.
“Ticks have a terrible reputation – they are not very nice to look at, need to suck blood to survive and are responsible for transmitting bacteria that cause severe diseases, such as Lyme disease in humans,” said Professor Richard Payne in the School of Chemistry.
“However, to a medicinal chemist, ticks are amazing creatures.”
Ticks have evolved an impressive arsenal of biologically active salivary proteins they pump into the bite sites on their hosts. Among these are various pain-killing agents and some of the best blood-thinning molecules known.
“In order to avoid detection, ticks also produce small protein molecules that suppress the inflammatory response. These proteins are called the evasins because they help the tick evade immune detection. This means they can feed for days without the host knowing they are attached,” Professor Payne said.
The proteins do this by binding to inflammatory molecules found within the blood called chemokines. In a normal infection or bite, the chemokines act as transmitters, letting the body know that white blood cells are needed to fight potential disease. The evasins therefore prevent the chemokines from causing inflammation at the bite site.
POWERFUL BIOLOGICAL KICK
In another first in her paper, lead author Charlotte Franck discovered that sulfate molecules attached to evasins give the proteins a powerful kick.
“Armed with this knowledge, evasins could potentially be repurposed to suppress chemokine-driven inflammation in human disease,” she said.
PhD candidate Ms Franck said: “We’ve known about evasins in tick spit for more than a decade, but my discovery has shown that the proteins are modified with sulfate groups. It is these modified forms of the protein that provide a massive enhancement in biological activity.”
When you try to isolate the evasins from the tick saliva, you end up with a big mixture of other molecules, making the evasins very difficult to test or reproduce.
“To overcome this, we built the proteins from scratch using synthetic chemistry,” said Ms Franck, a student from Belgium. No one else has previously done this.
“We are now trying to engineer these sulfated evasin molecules to make them even more effective and more stable in blood,” she said. “We can then start to explore how effective they could be for a range of inflammatory conditions in the clinic.”
Evasin proteins without the sulfate modifications have been shown to have excellent anti-inflammatory properties in animal disease models of lung fibrosis and in colitis – a type of gut inflammation.
Chemokines have also been shown to be drivers of lung inflammation in COVID-19. The researchers say it will be worthwhile exploring how evasins might be used to treat these symptoms, but any practical application would be years in the future.
Professor Payne said: “It is entirely possible that our sulfated tick proteins, or modified variants of them, could find wide application for a number of inflammatory diseases in the future.”
The work joins that of other research into naturally occurring proteins and their applications being undertaken by a new national centre funded by the Australian Research Council.
The ARC Centre of Excellence for Innovations in Peptide and Protein Science (CIPPS) was established this year and is dedicated to discovering new proteins, decoding their biological functions and developing them for use in health, agriculture and industry.
Professor Payne is CIPPS Deputy Director. He said: “Life has had billions of years to exquisitely fine-tune proteins, such as evasins, for particular tasks. We have an amazing opportunity now to understand how they work and seek innovative ways to apply them to challenges in medicine and other areas to benefit humanity.”
Professor Payne was awarded the Malcolm McIntosh Prize for Physical Scientist of the Year at the 2016 Prime Minister’s Prizes for Science.
DOWNLOAD the research, photos of the researchers and an illustration at this link.
INTERVIEWS
Professor Richard Payne | [email protected]
School of Chemistry
Deputy Director ARC Centre for Innovations in Peptide and Protein Science
The University of Sydney
Charlotte Franck | [email protected]
PhD candidate, School of Chemistry
The University of Sydney
MEDIA ENQUIRIES
Marcus Strom | [email protected] | +61 423 982 485
DECLARATION
This research was supported by an Australian Research Council Discovery Project (DP190101526) and a National Health and Medical Research Council Project Grant (APP1140867).
Charlotte Franck is generously supported a John A. Lamberton Research Scholarship.
Media Contact
Marcus Strom
[email protected]
Original Source
https:/
Related Journal Article
http://dx.