Credit: Pavel Odinev / Skoltech
Researchers from the Skoltech Center for Energy Science and Technology (CEST) visualized the formation of a solid electrolyte interphase on battery-grade carbonaceous electrode materials using in situ atomic force microscopy (AFM). This will help researchers design and build batteries with higher performance and durability.
A solid electrolyte interphase (SEI) is a thin layer of electrolyte reduction products formed on the surface of a lithium-ion battery anode during several initial cycles. It prevents further electrolyte decomposition, stabilizing the electrode/electrolyte interface, and ensures a long battery life. Forming a SEI film takes time and energy, and its quality largely governs battery performance and durability: a poorly formed SEI results in rapid degradation of battery performance.
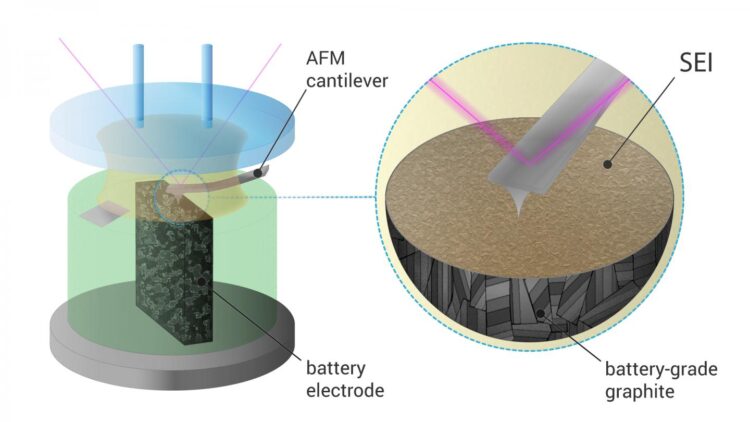
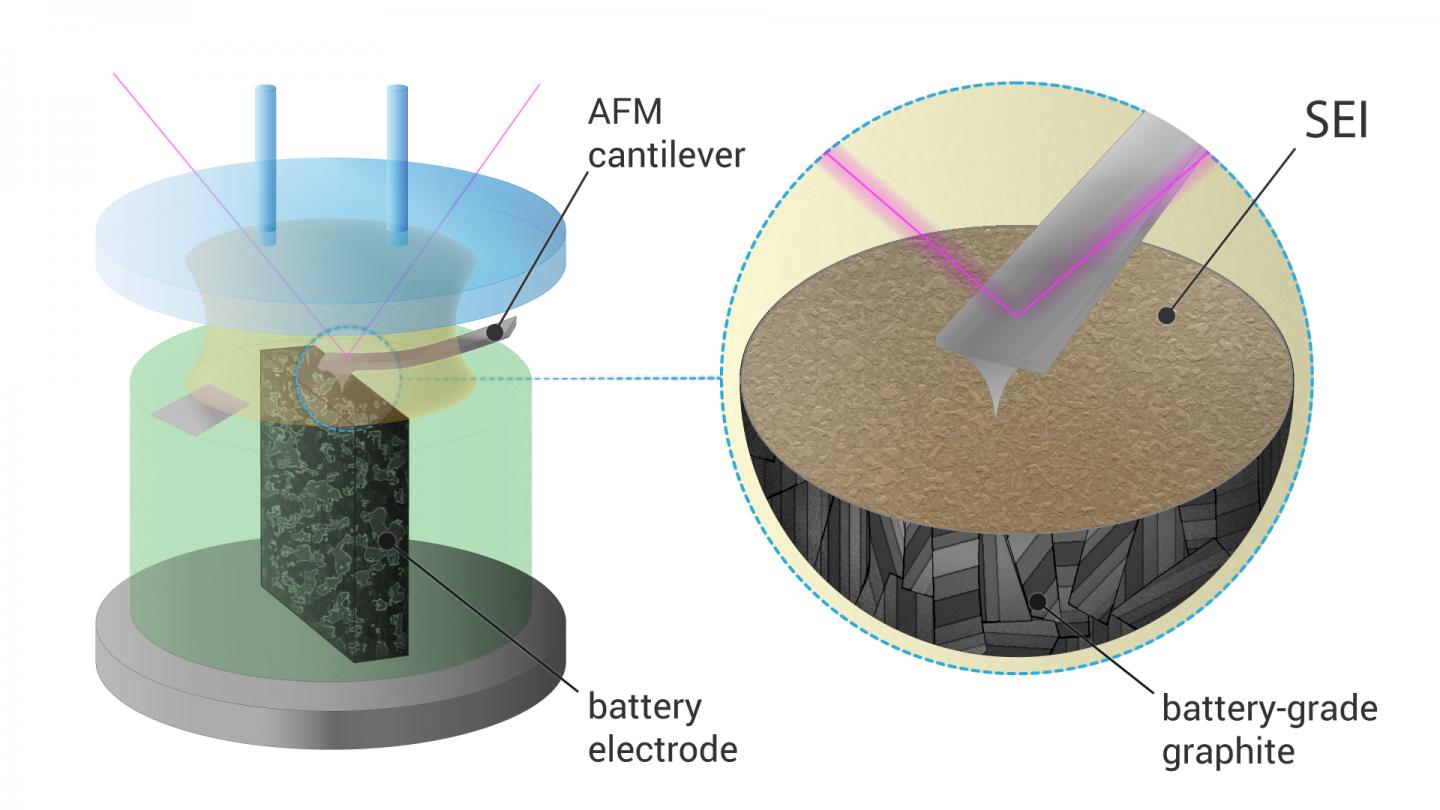
Still, the formation of SEI remains poorly understood, and scientists use in situ atomic force microscopy that allows direct observation of this process. Until now, most of these measurements were carried out on Highly Oriented Pyrolytic Graphite (HOPG), a very pure and ordered form of graphite which has a clean and atomically flat basal plane surface. However, HOPG is a poor replacement for actual battery-grade electrode materials, so the process is significantly different from what happens inside a commercial battery.
A Skoltech team led by research scientist Sergey Luchkin and professor Keith Stevenson succeeded in visualization of SEI formation on battery-grade materials. For this, they had to design an electrochemical cell that allowed the measurements necessary for this direct observation of SEI formation.
“Battery-grade materials are powders, and visualizing dynamic processes on their surface by AFM, especially in liquid environment, is challenging. A standard battery electrode is too rough for such measurements, and isolated particles tend to detach from substrate during scanning. To overcome this issue, we embedded the particles into epoxy resin and made a cross section, so the particles were firmly fixed in the substrate,” says Luchkin.
The researchers found that the SEI on battery-grade materials nucleated at different potential than that on HOPG. It was also more than two times thicker and mechanically stronger. Finally, they were able to demonstrate that SEI was better bound with the rough surface of battery-grade graphite than with the flat surface of HOPG.
“Spatially-resolved investigations of battery interfaces and interphases detailed in this work provide significant new insights into the structure and evolution of the anode SEI. Therefore, they provide firm guidelines for rational electrolyte design to enable high performance batteries with improved safety,” adds Stevenson.
###
Media Contact
Alina Chernova
[email protected]
Related Journal Article
http://dx.