Credit: Nakada Laboratory, Waseda University
An anticancer drug of fungal origin could be the way.
Scientists at Waseda University succeeded in developing a method for a total synthesis of cotylenin A, a plant growth regulator which has attracted considerable attention from the scientific community due to its promising bioactivity as an anti-cancer agent. This method was reported in the Journal of the American Chemical Society on March 16, 2020.
“Our method will be able to provide steady supply of cotylenin A, which could possibly lead to the development of an effective anticancer drug,” says Masahisa Nakada, a professor of synthetic chemistry at Waseda University in Tokyo, Japan and corresponding author of this study.
Nakada believes that this is the world’s first total synthesis of cotylenin A to ever be reported.
Previous biological studies have revealed that cotylenin A combined in treatment can program cell death for a wide range of human cancer cell lines and suppress tumor growth. In addition, cotylenin A was found to induce the differentiation of myeloid leukemia cells when combined with a specific pharmaceutical drug.
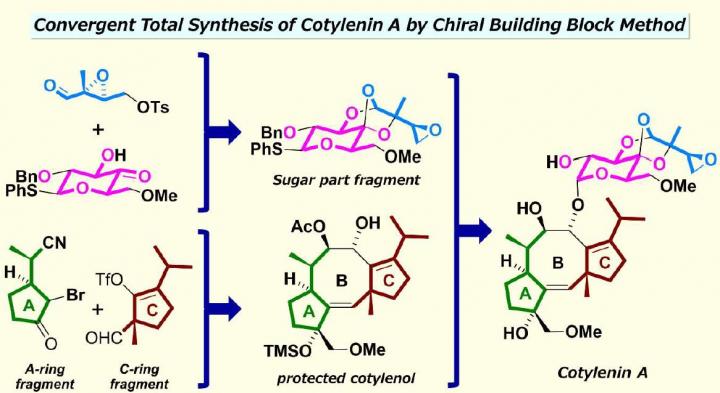
Despite its potential, producing cotylenin A from natural resources is not possible because the fungus which produces cotylenin A loses all its ability to proliferate during preservation, which would create a dearth of supply. Also, cotylenin A has a complex structure where two carbocyclic five-membered rings are fused with a formidable carbocyclic eight-membered ring with a uniquely-structured, glucose-based sugar attached. Chemically synthesizing a carbocyclic eight-membered ring is known to be difficult, and because the carbocyclic ring system includes a number of functional groups such as moieties, these features have made it challenging for scientists in the field to artificially synthesize cotylenin A.
“What we did in our method was to separate the structure of cotylenin A into three fragments,” Nakada explains. “Each fragment was then prepared with commercially-available chemical compounds using different reactions, such as a catalytic asymmetric intramolecular cyclopropanation (CAIMCP) developed by us. Afterwards, we assembled the two fragments back together using a coupling reaction, followed by a cyclization of the carbocyclic eight-membered ring which was mediated by the chemical element palladium and a coupling with the sugar-part fragment which was catalyzed by the chemical element rhodium.” He adds, “The total synthesis took about 25 steps, and our synthesized product is identical to those of naturally occurring cotylenin A.”
Nakada says that his team has now succeeded in achieving this synthesis in only 20 steps, and by further reducing the number of steps, producing an adequate amount of cotylenin A will become possible. He hopes that the method they developed will contribute to further biological studies of cotylenin A and to the discovery of an anticancer drug that exhibits stronger anticancer activity and causes no side effects by modifying cotylenin A, as well as to the application for a total artificial synthesis of various compounds in the future.
###
Reference
Title of original article: Enantioselective Total Synthesis of Cotylenin A
Authors: Masahiro Uwamori, Ryunosuke Osada, Ryoji Sugiyama, Kotaro Nagatani, and Masahisa Nakada
DOI: 10.1021/jacs.0c01774
Journal: Journal of the American Chemical Society
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university which has long been dedicated to academic excellence, innovative research and civic engagement at both the local and global levels since 1882. Today, the student body at Waseda is approximately 50,000, nearly 8,000 of whom are from overseas, hailing from 125 countries. To learn more about Waseda University, visit https:/
Media Contact
Jasper Lam
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




