Credit: Source: Faculty of Physics, University of Warsaw
Gene therapy gives hope to millions of patients. Researchers from the University of Warsaw have been working on mRNA containing a modified fragment which initiates protein biosynthesis. Recently published results reveal that new compounds – designed and synthesized at the University of Warsaw – are more stable and effective than their natural equivalents, and their synthesis is simpler. The compounds allow scientists to gain a better understanding of the mechanisms of protein biosynthesis in cells, which in turn should help them design better therapeutics.
Protein production is frequently disrupted in the cells of a disease-affected organism. This manifests as imbalance in the synthesis of certain proteins or production of damaged proteins, which in extreme cases leads to cancer. Gene therapy is one of the methods used for dealing with this problem. It involves supplying the organism with genetic material encoding proteins whose properties support healthy cell activity. In the early days of experiments on gene therapy, researchers used DNA as the genetic material. However, genes delivered in the form of DNA integrate with the patients' genome, which beside solving the targeted problem, may also bring new serious and unexpected symptoms. Medical researchers have high hopes as to the therapeutic potential of mRNA; the molecules are smaller and simpler, which makes them easier to prepare under laboratory conditions, and – perhaps most importantly – unlike DNA, they don't make permanent changes to the organism's genetic makeup.
mRNA molecules are natural polymers formed in cells. They contain precise copes of genes (DNA fragments), so they carry the genetic code and act as templates in the production of new proteins. mRNA molecules are broken down by enzymes after a few minutes or hours. This short lifespan of natural and synthetic mRNA is one of the problems limiting its practical applications. The application of mRNA in gene therapy would be more feasible, if the molecule used in drugs "survived" for longer than its natural equivalent, and if the therapeutic efficacy was as high as possible.
The team working on mRNA stabilization was originally founded at the Division of Biophysics (Institute of Experimental Physics, Faculty of Physics, the University of Warsaw). The initiator of research into mRNA structure and function is Prof. Edward Darzynikiewicz (Faculty of Physics, University of Warsaw). The team working on therapeutic modifications of mRNA molecules is led by Prof. Jacek Jemielity, formerly from the Faculty of Physics at the University of Warsaw and currently working at the Centre of New Technologies at the University of Warsaw. The team brings together over a dozen PhD holders and student researchers, with Dr. Joanna Kowalska acting as the main animator of research at the University of Warsaw. The scientists work alongside colleagues from the US and Germany and with pharmaceutical companies. In 2011, the consortium between the University of Warsaw and the Louisiana State University patented and commercialized the team's invention improving the stability and efficiency of mRNA. The solution is currently undergoing clinical tests conducted by one of the pharma partners. The key innovation is the five-prime cap (5' cap) – an artificial mRNA segment replacing its natural 7-methylguanosine structure.
The ongoing Warsaw research aims to discover new, better cap analogues, design a technology for large-scale production of therapeutic mRNA, and improve understanding of the course of natural protein synthesis. "The 7-methylguanosine cap is at the 5' end of the mRNA molecule," explains Prof. Darzynkiewicz. "In cytoplasm, the cap structure is recognized by the eIF4E factor which initiates the process of protein biosynthesis, known as translation. This stage decides on the speed of the entire complex sequence of events, which culminate with the synthesis of protein in the cell. The cap protects the mRNA from degradation by cleaving enzymes – nucleases. Unfortunately cells remove the cap using decapping enzymes such as Dcp1/Dcp2. A few years ago we discovered that using modified cap analogues can prevent the degradation of the 5' mRNA end and improve the rate of translation."
The team's latest results reveal that the search for new, natural cap analogues is promising. "Our recent paper, published in Nucleic Acids Research,(1) presents a new class of modified caps which are an improved version of those currently undergoing clinical trials," says Prof. Jemielity. "The modification involves swapping an oxygen atom for a sulfur atom in several positions in a specific place of the cap molecule, known as the tri- or tetra-phosphate bridge. mRNA with this chemically modified cap is bound effectively by the eIF4E factor during the stage limiting the speed of protein biosynthesis. It's also highly resistant to the cleaving of the cap structure by the Dcp1/Dcp2 enzyme. Under cellular conditions this mRNA is more stable and produces higher amounts of therapeutic protein, which we have demonstrated in a model used in studies of cancer vaccines. We hope that our modified mRNA will enable us to use lower doses of therapeutics – and lower doses mean a lower risk of side effects."
The drug's availability is also very important for the patient. Traditional enzyme methods of modifying caps (and in turn therapeutic mRNA) are time consuming and very ineffective. "Back in 2010, it took us six months to prepare the first four grams of cap needed to start clinical trials, and the amount was barely sufficient to treat 12-13 patients," recalls Dr. Kowalska. Meanwhile, the potential demand can be estimated as kilograms of the compounds every year, leading researchers to seek faster and cheaper production methods. "We turned our attention to click chemistry," says Sylwia Walczak, PhD student at the University of Warsaw. "We have been developing a method of effective synthesis of cap analogues from prefabricated units – chemical 'building blocks'. The structure of each block has at least one fragment which joins its counterpart in another molecule, interlocking like bricks." By applying the method in straightforward production of modified caps, scientists from Poland have developed 36 new analogues. "Two of the compounds have properties we were hoping for: when they are introduced to mRNA, they work as well as the natural cap," adds Anna Nowicka, working on her PhD at the University of Warsaw. "We are certain that this discovery will pave the way to developing new chemical methods of adding the cap to mRNA which will compete with expensive and time consuming enzymatic methods," adds Nowicka. The work of the team of eight authors describing the discovery was published in late summer in the leading journal Chemical Science.(2)
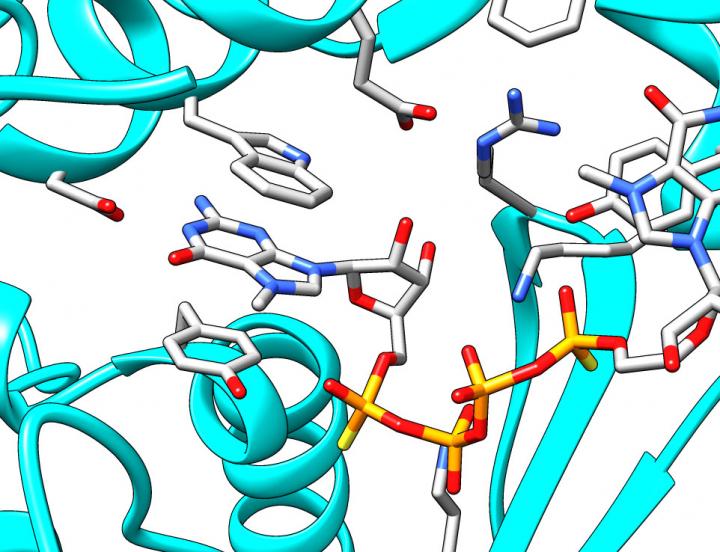
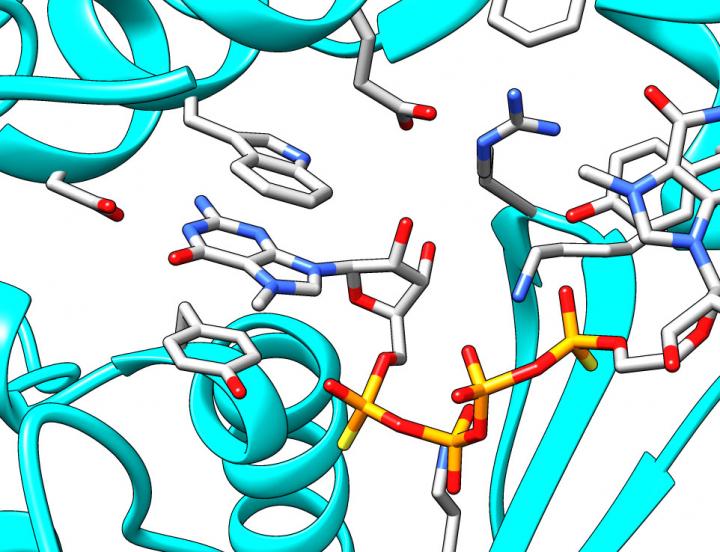
The search for new, improved cap analogues slowly shifts from the trial and error approach towards rational design. This is possible due to advances in the understanding of mRNA-related processes, their control and dynamics. Recently, the team from Warsaw contributed to new insights into mRNA decapping "For the first time we have been able to design compounds which, by mimicking the 5' mRNA cap, are able to inhibit the Dcp1/Dcp2 enzyme, which cleaves the cap from mRNA exposing it to degradation," says Dr. Marcin Ziemniak, who completed his PhD at the Faculty of Physics at the University of Warsaw earlier this year. "Working with colleagues at the University of California in San Francisco, John D. Gross and Jeffrey Mugridge, we have used X-ray crystallography to get new insight into structure and function of Dcp1/Dcp2. We have used our compound to capture the key stage of enzyme activity, which is binding the cap. To put it more simply, we used our compound as a bait, which imitates the mRNA cap. The enzymatic complex 'swallows' the bait, 'freezes', and can be 'photographed'. Our results indicate that as the bait is taken – the inhibitor is bound – the enzyme complex undergoes global structural changes. The chemical composition of molecules remains unchanged, of course, but their fragments rotate relative to one another to reach a situation when the enzyme is ready to act." The results have been published in two prestigious journals: RNA (January) and Nature Structural and Molecular Biology (October).(3,4) "We believe that the results will allow us to design even better inhibitors of mRNA decapping," stresses Prof. Jemielity. "They will be useful in further research into mRNA degradation processes, and hopefully they will also find therapeutic applications such as increasing the potency mRNA-based gene therapies."
Scientists stress that the problems they are working on require an interdisciplinary approach. "The work we are conducting at the Faculty of Physics is unique," says Dr. Kowalska. "We have access to state-of-the-art research labs, although it's true to say that other teams have similar equipment. Our advantage lies in our team, which consists from experts in biophysics, chemistry and molecular and cellular biology. Conducting research on the boundaries of three different disciplines and the ability to look at the same research problem from different perspectives is incredibly inspirational, and gives us opportunity to come up with completely fresh ideas and solutions which would be far more difficult to reach using just a single approach. I believe this is a unique approach not only in Poland but on a global scale," Kowalska sums up the situation.
###
Physics and Astronomy first appeared at the University of Warsaw in 1816, under the then Faculty of Philosophy. In 1825 the Astronomical Observatory was established. Currently, the Faculty of Physics' Institutes include Experimental Physics, Theoretical Physics, Geophysics, Department of Mathematical Methods and an Astronomical Observatory. Research covers almost all areas of modern physics, on scales from the quantum to the cosmological. The Faculty's research and teaching staff includes ca. 200 university teachers, of which 88 are employees with the title of professor. The Faculty of Physics, University of Warsaw, is attended by ca. 1000 students and more than 170 doctoral students.
SCIENTIFIC PAPERS:
1. "Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential", By Malwina Strenkowska, Renata Grzela, Maciej Majewski, Katarzyna Wnek, Joanna Kowalska, Maciej Lukaszewicz, Joanna Zuberek, Edward Darzynkiewicz, Andreas N Kuhn, Ugur Sahin, Jacek Jemielity, Published in Nucleic Acids Research 44 (2016) doi: 10.1093/nar/gkw896, http://nar.oxfordjournals.org/content/early/2016/10/06/nar.gkw896.full
2. "A novel route for preparing 5? cap mimics and capped RNAs: phosphate-modified cap analogues obtained via click chemistry", By Sylwia Walczak, Anna Nowicka, Dorota Kubacka, Kaja Fac, Przemyslaw Wanat, Seweryn Mroczek, Joanna Kowalska, Jacek Jemielity, Published in Chemical Science 7 (2016) DOI: 10.1039/C6SC02437H, http://pubs.rsc.org/en/content/articlelanding/2014/SC/C6SC02437H#!divAbstract
3. "Two-headed tetraphosphate cap analogs are inhibitors of the Dcp1/2 RNA decapping complex", By Marcin Ziemniak, Jeffrey S. Mugridge, Joanna Kowalska, Robert E. Rhoads, John D. Gross, Jacek Jemielity, Published in RNA 22 (2016) 518-529, http://rnajournal.cshlp.org/content/early/2016/01/29/rna.055152.115
4. "Structural basis of mRNA-cap recognition by Dcp1-Dcp2", By Jeffrey S. Mugridge, Marcin Ziemniak, Jacek Jemielity, John D. Gross, Published in Nature Structural & Molecular Biology 23 (2016) doi:10.1038/nsmb.3301, http://www.nature.com/nsmb/journal/vaop/ncurrent/full/nsmb.3301.html
CONTACTS:
Dr. Joanna Kowalska
Institute of Experimental Physics, Faculty of Physics, University of Warsaw
tel. +48 22 55 40 774, +48 22 55 40 788
email: [email protected]
Prof. Edward Darzynkiewicz
Institute of Experimental Physics, Faculty of Physics, University of Warsaw
tel. +48 22 55 40 787
email: [email protected]
Prof. Jacek Jemielity
Centre of New Technologies, University of Warsaw
+48 22 55 43774
e-mail: [email protected]
RELATED LINKS:
http://www.jemielitygroup.pl/
http://www.biogeo.uw.edu.pl
http://www.fuw.edu.pl/
Faculty of Physics, University of Warsaw.
http://www.fuw.edu.pl/informacje-prasowe.html
Press office of the Faculty of Physics, University of Warsaw.
IMAGES:
FUW161109b_fot01s.jpg
HR: http://www.fuw.edu.pl/press/images/2016/FUW161109b_fot01.jpg
Fragment of the X-ray crystal structure showing the cap bound by Dcp1/Dcp2. Based on pdb-entry 5KQ4. (Source: Faculty of Physics, University of Warsaw)
Media Contact
Dr. Joanna Kowalska
[email protected]
48-225-540-774
http://www.fuw.edu.pl
############
Story Source: Materials provided by Scienmag