Credit: Argonne National Laboratory
The diamonds and other crystals on view in science museum exhibits are a delight to the eye. What contributes to their sometimes dazzling geometric shapes and colors is their highly ordered arrangement of atoms. For the crystalline materials in battery electrodes, their ordered microstructure has practical benefits for ease of the ion transfer within the electrode during charge and discharge.
Scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have created and tested a single-crystal electrode that promises to yield pivotal discoveries for advanced batteries under development worldwide for electric vehicles, consumer electronics and other applications. Collaborating on the project were researchers from Northwestern University and the University of Illinois at Chicago.
“We recognized that single crystals can play a vital role in identifying promising new ways to understand, at atomic and molecular levels, the chemistries that control charge-discharge processes in batteries with polycrystalline electrodes.” — Sanja Tepavcevic, Argonne’s Materials Science division
The electrode materials in advanced batteries are “polycrystalline,” meaning they have numerous differently oriented crystalline regions. Because polycrystalline electrodes are relatively simple to fabricate, scientists have focused past battery research on experimenting with these materials, which are full of different kinds of defects within the ordered structures that often can affect performance.
“We recognized that single crystals can play a vital role in identifying promising new ways to understand, at atomic and molecular levels, the chemistries that control charge-discharge processes in batteries with polycrystalline electrodes,” noted Sanja Tepavcevic, assistant scientist in Argonne’s Materials Science division.
As a model system to investigate their single-crystal cathode, the team chose the sodium-ion battery under development to compete with current lithium-ion batteries. The main attraction of these batteries is that sodium is far more abundant an element than the lithium used for lithium-ion batteries.
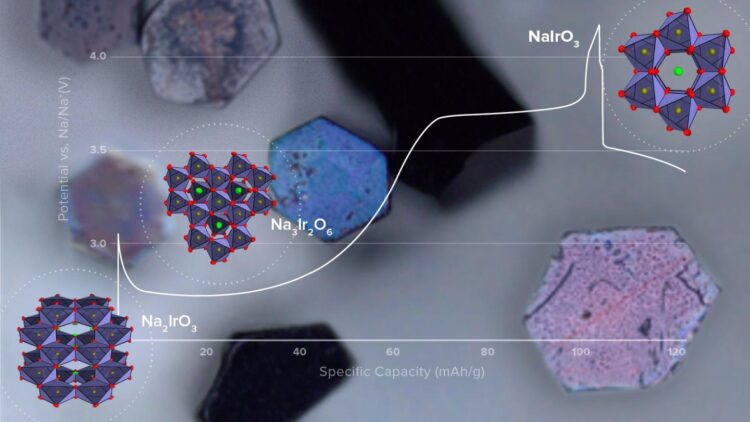
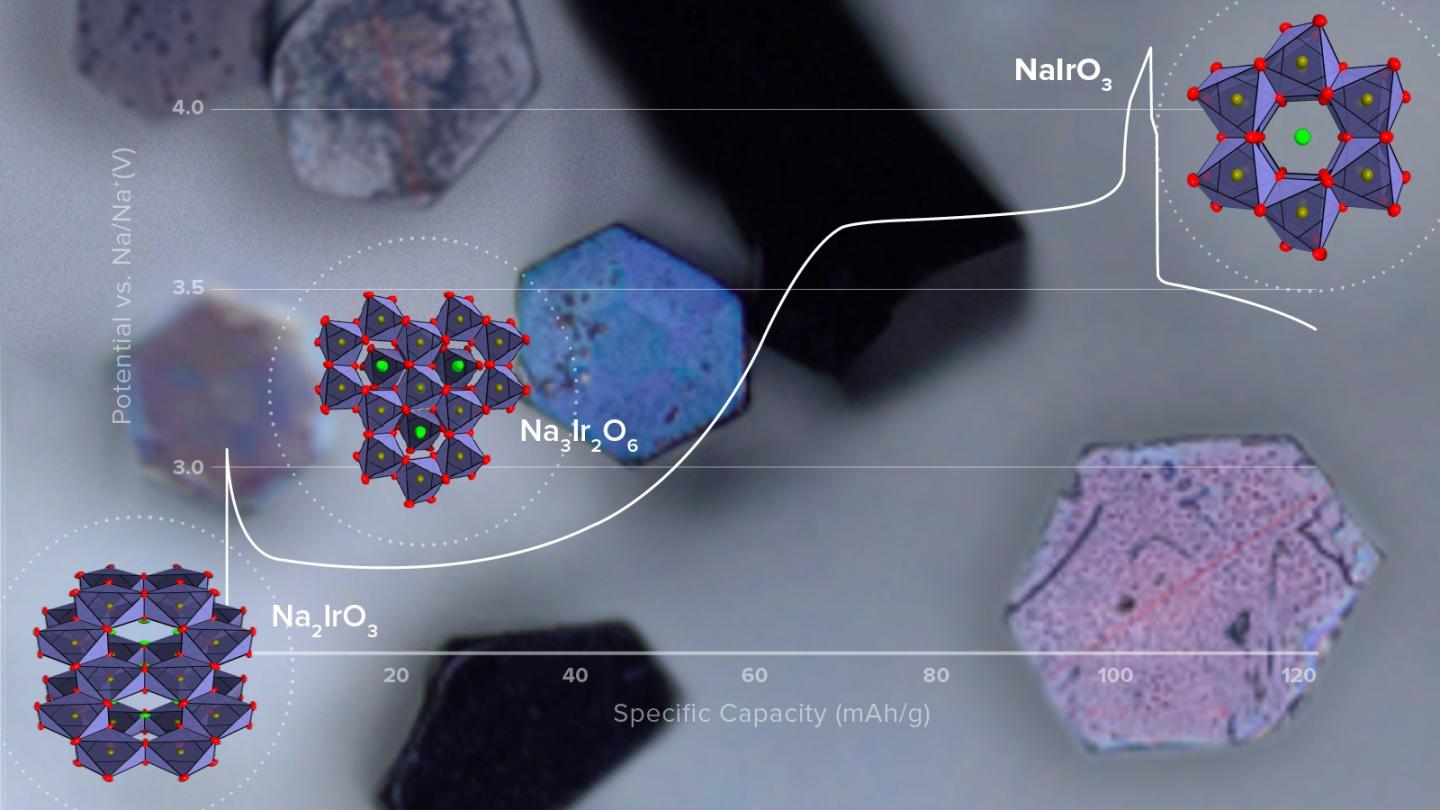
The team prepared single crystals of a sodium-iridium oxide (Na2IrO3) and used them as the cathode material in small test cells. For comparison, they also tested similar cells with polycrystalline cathodes. By drawing upon the scientific facilities at Argonne — in particular, the Advanced Photon Source (APS), a DOE Office of Science User Facility — they could determine the precise position of every atom in the crystal structure for different states of cell charge and discharge.
“This project simply would not have been possible without the extraordinary material characterization resources of the APS,” said Tepavcevic. “We also greatly benefitted from the expertise of team member Jennifer Hong Zheng in her world-class capability at growing single crystals to precise specifications.”
Much was learned about the cathode chemistry during charge-discharge cycling of the test cells. In particular, the team investigated the origin of the extra capacity beyond that expected for the NaIrO3 endpoint structure. “With our single crystals, we could separate surface from bulk effects that were not apparent in earlier work with polycrystalline materials alone,” said Tepavcevic. The team demonstrated that the extra capacity derives from surface reactions, not the bulk of the material as previously thought.
Important to improved battery design is knowing how and why material changes occur during cycling. From their test results, the team determined the chemical structure of the three distinct phases that form during charge, two of which were not known before. They also found that cell capacity faded with cycling because of the formation of a new detrimental phase on charge, which persisted during discharge and grew in size with cycle number.
“We learned more about sodium-ion batteries with our single-crystal electrodes than we ever thought possible at the project start,” said John Mitchell, Argonne Distinguished Fellow in the Materials Science division. “Clearly, single crystals open the window to a far better understanding of the chemical and electronic transformations that control energy storage and release in all battery types, as well as their degradation mechanisms with cycling.” With such knowledge, future battery researchers will be able to develop design rules for synthesizing new and improved polycrystalline materials with desired functionality.
###
This study, titled “Fundamental insights from a single-crystal sodium iridate battery,” appeared in Advanced Energy Materials print edition (March 2020). In addition to Tepavcevic, Zheng and Mitchell, Argonne authors include D.G. Hinks, Baris Key, Yang Ren, J.W. Freeland and N.M. Markovic. University collaborators include Logan Ward, Zhi Lu, Costas Stoumpos and Christopher Wolverton from Northwestern University and Patrick Philips and Robert Klie from the University of Illinois at Chicago. This study received funding from the U.S. DOE Office of Basic Energy Sciences and U.S. Department of Commerce, as well as support through the Joint Center for Energy Storage Research.
About the Advanced Photon Source
The U. S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. The APS provides high-brightness X-ray beams to a diverse community of researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. These X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being. Each year, more than 5,000 researchers use the APS to produce over 2,000 publications detailing impactful discoveries, and solve more vital biological protein structures than users of any other X-ray light source research facility. APS scientists and engineers innovate technology that is at the heart of advancing accelerator and light-source operations. This includes the insertion devices that produce extreme-brightness X-rays prized by researchers, lenses that focus the X-rays down to a few nanometers, instrumentation that maximizes the way the X-rays interact with samples being studied, and software that gathers and manages the massive quantity of data resulting from discovery research at the APS.
This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https:/
Media Contact
Diana Anderson
[email protected]
Original Source
https:/
Related Journal Article
http://dx.