Credit: © 2020 Mawadda Alghrably
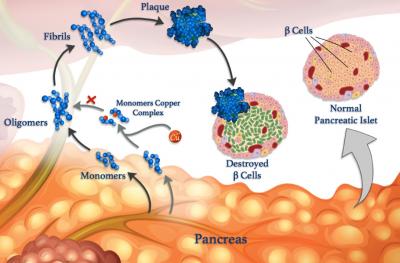
Unraveling interactions between metal ions and peptides in the body may eventually lead to improved treatments for diabetes, Alzheimer’s and other diseases. Understanding these interactions is the focus of research, co-led by KAUST, that is revealing how metals, such as copper, can affect the formation of harmful clumps of misfolded peptide clusters called fibrils, which underpin many diseases.
Errant peptides are linked to neurological conditions such as Alzheimer’s, as well as to blood sugar control disease diabetes. Blood sugar levels are normally controlled via peptide hormones released by specialist cells called β-cells. As well as insulin, healthy β-cells also release amylin, a peptide hormone that helps to reduce spikes in blood sugar level after eating by slowing stomach emptying. But amylin is prone to forming misfolded clumps, especially in the presence of copper ions, that damage β-cells and contribute to type II diabetes.
However, metal ions can also counteract peptide aggregation in some circumstances, says KAUST researcher, Mariusz Jaremko, who led the work in collaboration with researchers from the University of Wroclaw in Poland. To study the process in more detail, the team is examining the interaction between copper(II) ions and amylin and its molecular analogs. “Such knowledge would give us insights into the molecular mechanisms of type II diabetes, enabling us to design new strategies and therapies against this disease,” Jaremko says.
In their latest work, the team studied the influence of copper ions on the aggregation of two analogs of human amylin: an amylin-mimicking drug called pramlintide and amylin from rats. “We found that differences in the structures of pramlintide and rat amylin mean that copper ions impede the aggregation of pramlintide, but not rat amylin,” says Mawadda Alghrably, a Ph.D. student in Jaremko’s team.
The researchers investigated the process using multiple techniques, including nuclear magnetic resonance (in collaboration with Abdul-Hamid Emwas from KAUST CoreLabs), and a “thioflavin T” fluorescence assay of protein aggregation. They found that although both amylin analogs bind copper, pramlintide could bind it in two different ways due to an extra copper-binding histidine amino acid that is present in pramlintide but not rat amylin. Copper-ion binding to this histidine probably explained why copper reduced pramlintide aggregation but not rat amylin aggregation, the researchers concluded.
The team is continuing to decipher the molecular basis of amylin aggregation, Alghrably says. “Understanding how these molecules behave, could ultimately help to facilitate the design of new efficient drugs and therapies for type II diabetes,” she says.
###
Media Contact
Carolyn Unck
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




