Credit: Nagahiro Saito
Nagoya University scientists have developed a one-step fabrication process that improves the ability of nanocarbons to remove toxic heavy metal ions from water. The findings, published in the journal ACS Applied Nano Materials, could aid efforts to improve universal access to clean water.
Various nanocarbons are being studied and used for purifying water and wastewater by adsorbing dyes, gases, organic compounds and toxic metal ions. These nanocarbons can adsorb heavy metal ions, like lead and mercury, onto their surfaces through molecular attraction forces. But this attraction is weak, and so they aren’t very efficient adsorbents on their own.
To improve adsorption, scientists are considering adding molecules to the nanocarbons, like amino groups, that form stronger chemical bonds with heavy metals. They are also trying to find ways to use all available surfaces on nanocarbons for metal ion adsorption, including the surfaces of their inner pores. This would enhance their capacity to adsorb more metal ions at a time.
Materials scientist Nagahiro Saito of Nagoya University’s Institute of Innovation for Future Society and colleagues developed a new method for synthesizing an “amino-modified nanocarbon” that more efficiently adsorbs several heavy metal ions compared to conventional methods.
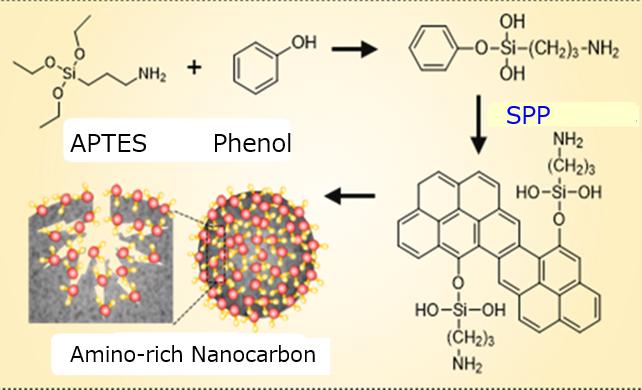
They mixed phenol, as a source of carbon, with a compound called APTES, as a source of amino groups. This mixture was placed in a glass chamber and exposed to a high voltage, creating a plasma in liquid. The method they used, called “solution plasma process,” was maintained for 20 minutes. Black precipitates of amino-modified carbons formed and were collected, washed and dried.
A variety of tests showed that amino groups had evenly distributed over the nanocarbon surface, including into its slit-like pores.
“Our single-step process facilitates the bonding of amino groups on both outer and inner surfaces of the porous nanocarbon,” says Saito. “This drastically increased their adsorption capacity compared to a nanocarbon on its own.”
They put the amino-modified nanocarbons through ten cycles of adsorbing copper, zinc and cadmium metal ions, washing them between each cycle. Although the capacity to adsorb metal ions decreased with repetitive cycles, the reduction was small, making them relatively stable for repetitive use.
Finally, the team compared their amino-modified nanocarbons with five others synthesized by conventional methods. Their nanocarbon had the highest adsorption capacity for the metal ions tested, indicating there are more amino groups on their nanocarbon than the others.
“Our process could help reduce the costs of water purification and bring us closer to achieving universal and equitable access to safe and affordable drinking water for all by 2030,” says Saito.
###
The article, “Liquid-Phase Plasma-Assisted in Situ Synthesis of Amino-Rich Nanocarbon for Transition Metal Ion Adsorption,” was published in the journal ACS Applied Nano Materials on December 27, 2019, at DOI:10.1021/acsanm.9b01915.
This work was supported by JST-SICORP Grant JPMJSC18H1 and JST-OPERA Grant JPMJOP1843.
About Nagoya University, Japan
Nagoya University has a history of about 150 years, with its roots in a temporary medical school and hospital established in 1871, and was formally instituted as the last Imperial University of Japan in 1939. Although modest in size compared to the largest universities in Japan, Nagoya University has been pursuing excellence since its founding. Six of the 18 Japanese Nobel Prize-winners since 2000 did all or part of their Nobel Prize-winning work at Nagoya University: four in Physics – Toshihide Maskawa and Makoto Kobayashi in 2008, and Isamu Akasaki and Hiroshi Amano in 2014; and two in Chemistry – Ryoji Noyori in 2001 and Osamu Shimomura in 2008. In mathematics, Shigefumi Mori did his Fields Medal-winning work at the University. A number of other important discoveries have also been made at the University, including the Okazaki DNA Fragments by Reiji and Tsuneko Okazaki in the 1960s; and depletion forces by Sho Asakura and Fumio Oosawa in 1954.
Media Contact
Nagahiro Saito
[email protected]
Original Source
http://en.
Related Journal Article
http://dx.




