Researchers at the UPV/EHU-University of the Basque Country have developed the technology needed to be able to determine the structure of sugars present in DNA
Credit: Emilio J. Cocinero
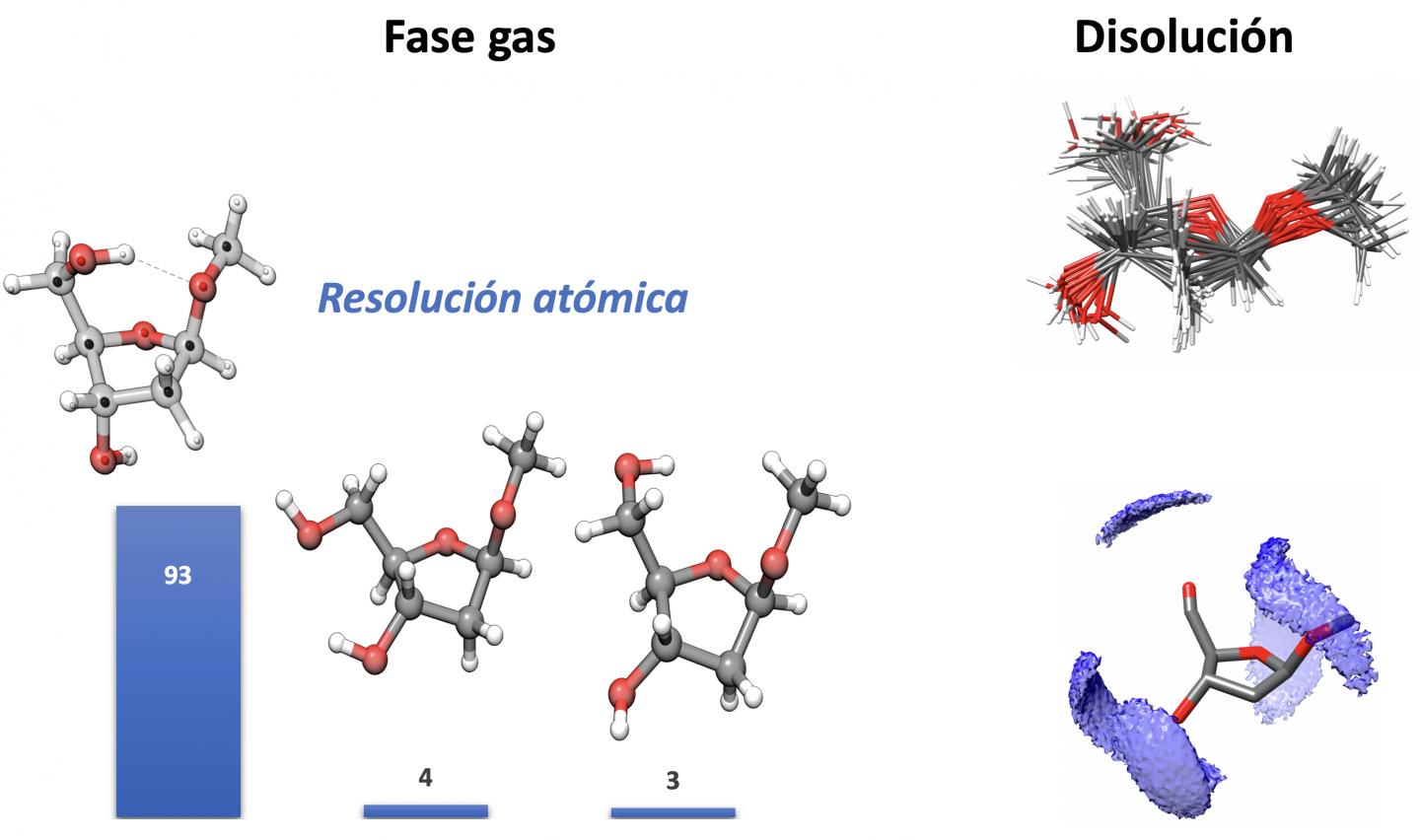
A piece of research work conducted by the Spectroscopy Group of the UPV/EHU’s Department of Physical Chemistry, and the Biofisika Institute provides the cover of the latest issue of the ACS Central Science journal, which is one of the three leading journals in all areas of Chemistry. This research group has managed to determine the structure of the sugars that form part of DNA, 2-deoxyriboside, with atomic-level resolution. What has been achieved here is “unprecedented resolution; we have managed to spatially position each of the atoms of this sugar”, as described by the group’s leader Emilio J. Cocinero.
Cocinero regards this outcome as the culmination of a piece of work that has taken them over ten years: “This outcome has been made possible thanks to the increase in the sensitivity of the microwave spectrometer that we have in our group, which we designed, built and adapted ourselves and which right now is among the 3 best devices of this type in the world.”
One of the main hurdles they had to overcome was the huge variability and flexibility between the various forms or conformations that may be adopted by 2-deoxyriboside molecules. The atoms that form these sugar molecules can be organised by forming five-membered or six-membered rings. “In nature, biological forms display five-membered rings, but in the experiments when the sugar is completely isolated and removed from any solvent and without it interacting with the remaining elements that make up the DNA and determine its configuration, the most stable form of sugars that we were achieving were six-member rings,” explained Cocinero.
To resolve this situation they had the collaboration of researchers from the Department of Chemistry at Oxford University who helped them to synthesise the four forms that 2-deoxyribosides may adopt, both in their biological forms and in those that do not appear in nature, and they blocked them, “by adding a methyl group to the sugars to prevent some forms from interconverting into others, and to be able to study each of them individually”, specified the researcher.
That way, they were able to characterise in an isolated way the structure of each of them on an atomic scale, and afterwards with the help of researchers at the University of La Rioja they were able to analyse how the structure of these forms changes when they come into contact with the solvent, “which is more akin to the natural medium in which they are usually found. We saw the differences between some forms and others and characterised them”.
This analysis also enabled them to hypothesise “why the form that is observed in nature is the one that is observed and not another one. As we saw, the five-membered ring form is more flexible and the conformation it adopts in the DNA chain encourages the bonding of the consecutive nucleotides”, he said.
Now, armed with the instruments developed they are going to tackle “the study of larger molecules and try to build systems that are increasingly closer to actual biological forms to provide better responses. We are seeking the limit of technical instruments”, concluded Emilio J. Cocinero.
###
Media Contact
Matxalen Sotillo
[email protected]
34-688-673-770
Original Source
https:/
Related Journal Article
http://dx.