Credit: AMOLF
Researchers from AMOLF (Amsterdam, the Netherlands) and Harvard University (USA) show how the ability of organisms to move around plays a role in stabilizing ecosystems. In their paper published 19 February 2020 in Nature, they describe how the competition between ‘movers’ and ‘growers’ leads to a balance in which both types of bacteria can continue to exist alongside each other.
We are all too familiar with the threats to our planet’s ecosystems: global warming, forest fires, nitrogen deposition, biodiversity decline, and even mass extinctions. But what actually makes ecosystems stable or fragile? What prevents one species from outcompeting all others, and hence drive them to extinction?
These questions have captivated biologists since Darwin. We have learned that food-webs and cooperation between species are key pieces to this puzzle, because they help explain how species then depend on one another to survive. Now a group of biophysicists from the Netherlands and the US have advanced a startling finding: the active movement of organisms can also drive ecosystem diversity and stability – through a remarkably simple mechanism that does not require food-webs or cooperation.
“Movement is fundamental to essentially all organisms – even plants move by seed dispersal,” says Sander Tans at the AMOLF institute in Amsterdam. “Bacteria are well known to move actively. Our experiments show how this movement can keep different bacterial species, typically called strains, together in a larger population. There is a rich literature on the possible roles of movement in such coexistence of species, but direct experiments that can exclude other explanations were lacking.
The coexistence paradox – what prevents extinctions in a competitive world?
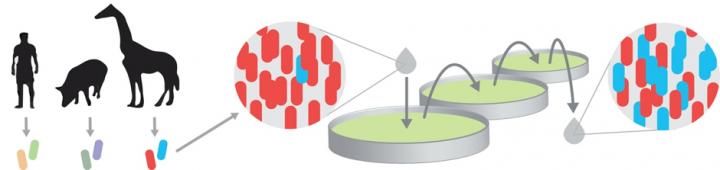
To find bacterial species that might form a minimal stable ecosystem in the lab, the PhD student Sebastian Gude took two species (also called strains) from the gut of the same animal. If both survived there, perhaps they would also do so in his experiments. To follow their competition, he colored one blue and the other red. However, all his first attempts failed in the beginning. One of the two strains always lost the competition when grown together, with these bacteria always producing fewer offspring than the other. This blue ‘loser’ strain would thus become outnumbered by the red ‘winner’ strain, and was ultimately driven to extinction.
Gude’s luck took a dramatic up-turn, however, when he changed the design of the experiment. He took the sugary liquid that the bacteria normally grew in, and turned it into a gel, reminiscent of a jelly desert. When the blue ‘loser’ bacteria became outnumbered by the reds when growing on this gel, they started to produce more offspring than the reds, and were thus winning. But in turn the reds also became more competitive when rare. In this way both strains thus escaped extinction, and hence coexisted together. These results underscore the fundamental paradox of the coexistence debate: what causes losers that are close to extinction to suddenly start winning?
Getting territorial
To resolve this conundrum, Gude followed the competition by making movies. “The results were quite striking,” says Tom Shimizu. “We saw the populations migrating into the gel like a wave, where they multiplied using the sugars they encountered. Initially, red dominated the expansion and blue was barely seen. But then the red advance suddenly stopped – just when the blues emerged and were seen to overtake the reds’ front, where they formed just a thin layer. After that, the wave was blue only. The blue bacteria could thus proliferate alone in the deeper regions of the gel, freed from competition from the reds that could not reach that far. This also explained the coexistence: whenever blue were rare, they could build up their population in the deeper regions of the gel.”
Should I spread or should I grow?
But how did the blue bacteria organize themselves and efficiently confine the red? Did they signal to each other or secrete toxins, as is known for some bacteria? In trying to address these questions the team discovered a rather different mechanism. The blue bacteria were indeed worse at proliferating, as they lost in a direct competition. But they compensated by migrating faster. By reaching the farther regions first, they could finish the local sugars. Hence, they gave the red no chance there, and could thus block their advance, a bit like in a scorched earth strategy.
Tans: “Some bacteria apparently are good at proliferating, and others at migrating. But they cannot excel at both. This makes sense because both activities cost a lot of energy. Such specialization is often observed, though effects on coexistence are often difficult to prove. Here we could manipulate the capacity to migrate and proliferate by genetic engineering, and show it alone is enough for coexistence. Other mechanisms like exchanging toxins or dependencies like in food-webs are thus not required per se.”
Disturbance ecology
Out in the real world, bacteria of course cannot count on encountering bowls of jello left out of the fridge. Luckily for them they don’t need to, as pristine pastures of fresh nutrients do arise more often than one might expect. Much like the vegetation that colonize cleared soil after forest fires, many bacteria grow on patches of resources: from windfallen fruits to decomposing animals, or – for those microbes that inhabit your gut – the lunch you just had. Shimizu: “We demonstrated this migration-proliferation mechanism in motile bacteria. But what we found is for instance evocative of models of plant ecology, where fast-growing plants compete with plants that invest more in spreading their seeds.” He added that there is a lot to be learned about more complex scenarios. “There is a lot of interest in explaining the diversity of bacteria in the human gut, which we now know, thanks to modern DNA analysis, is intimately related to our health. Our findings suggest that looking at motility genes and their spatial distribution within the gut might help explain some of that diversity.”
###
Media Contact
Sander J. Tans
[email protected]
31-207-547-255
Related Journal Article
http://dx.




