Credit: The Hyser lab
Some gastrointestinal viruses need calcium. They need calcium ions to carry out several essential aspects of viral life, such as entry into host cells, genome replication and building new viruses to invade other cells. The cells invaded by viruses also use calcium. They use it as signals to regulate many of the cells’ own processes, but viruses can takeover cellular calcium signaling to satisfy their own needs.
This viral takeover involves, in many cases, the production of a viral protein called viroporin that acts like an ion channel redirecting cellular calcium signaling to serve viral functions. Medically important gastrointestinal viruses, such as rotavirus, require calcium for replication and use viroporins to gain control over cellular calcium signaling, but it has not been investigated whether other viruses that also cause severe gastrointestinal problems in people and animals, such as norovirus, do the same.
Viroporins taking over?
Graduate student Alicia Strtak in the Graduate Program in Integrative Molecular and Biomedical Sciences at Baylor College of Medicine, took on the project to investigate whether Tulane virus, a calicivirus that is biologically similar to human noroviruses, required calcium for its replication. If so, how does it take over cellular calcium signaling?
“Human noroviruses are the leading cause of acute gastroenteritis, a potentially life-threatening illness in every age group,” said Strtak. “There is great interest in developing effective therapies, but many aspects of how calicivirus, including norovirus, cause disease have not been made clear yet.”
Studying norovirus has posed quite a challenge to researchers and, although much progress has been made to successfully culture it in the laboratory using human intestinal enteroid cultures, there is still much to be gained from studying other caliciviruses, such as Tulane virus, that are closely related to norovirus and are easier to study in the lab.
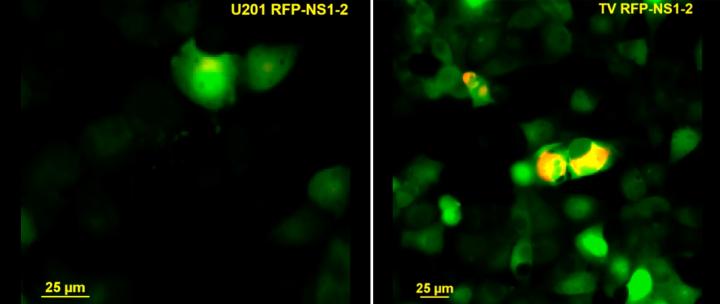
During this project, Strtak worked in the laboratory of Dr. Joseph Hyser, assistant professor of molecular virology & microbiology at Baylor, and used Tulane virus as a model system for norovirus.
“We think that Tulane virus has features that make it a good candidate for a model of human norovirus. These viruses share biologic and genetic characteristics, for instance, both organize their genomes in the same manner, infect the same type of cells (intestinal epithelia) and cause a disease the presents in a similar way,” Strtak said. “In addition, Tulane virus can be grown in the lab in systems that facilitate investigation into the pathways it takes over during infection.”
Requirements for replication
Strtak, Hyser and their colleagues combined cell culture, biochemistry and calcium imaging techniques to answer the following question, what are the key factors Tulane virus needs to achieve robust and efficient replication? To address the question, they focused on how Tulane virus reshaped the host’s calcium signaling during infection.
“First, we discovered that Tulane virus does need intracellular calcium to replicate. Without it, the virus replicates poorly,” Strtak said. “We also found that the calcium came from cellular storage in the endoplasmic reticulum and, to gain access to this source of calcium, Tulane virus seemed to use its protein NS1-2.”
The researchers found evidence that Tulane virus NS1-2 protein acted as a viroporin, an ion channel that disrupted cellular calcium signaling by triggering its flow from the endoplasmic reticulum, where it was stored, to the cytoplasm where viral replication took place.
Tulane virus findings hint at norovirus replication
Looking to determine whether norovirus might be using a similar strategy to infect epithelial cells, Strtak and her colleagues compared Tulane virus NS1-2 protein and the human norovirus protein in their ability to disrupt calcium signaling. They found that norovirus NS1-2 protein induced changes in cellular calcium signaling that were similar to those triggered by Tulane virus NS1-2 protein.
“This is the first piece of functional evidence suggesting that the function that we identified in the Tulane virus may also exist in human norovirus,” Strtak said.
“Viral ion channels are very difficult to identify. Strtak’s work shows that with a little bit of innovation, ingenuity and willingness to explore outside the clinically relevant system, it is possible to find a suitable experimental model that enables researchers to achieve the initial broad characterization and find relevant information about complex biological systems. We hope that the information we have found here also helps other researchers who study other aspects of viral infection,” said Hyser, a member of the Alkek Center for Metagenomics and Microbiome Research and the Dan L Duncan Comprehensive Cancer Center at Baylor.
###
Learn all the details of this work in the journal mSphere.
Other contributing authors include Jacob L. Perry, Mark N. Sharp, Alexandra L. Chang-Graham and Tibor Farkas. The authors are affiliated with one or more of the following institutions: Baylor College of Medicine, Louisiana State University School of Veterinary Medicine, Louisiana Animal Disease Diagnostic Laboratory and Augustana College.
Financial support was provided by National Institutes of Health grants (R01DK115507, R21AI137710, F30DK112563, DK56338 and CA125123) and Baylor College of Medicine’s Medical Scientist Training Program. Additional support came from the Integrative Molecular and Biomedical Sciences Graduate Program (T32GM008231), CPRIT (RP150578 and RP170719), the Dan L. Duncan Comprehensive Cancer Center and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.
Media Contact
Dipali Pathak
[email protected]
713-798-4710
Original Source
https:/
Related Journal Article
http://dx.



