Credit: Reza Shahbazian-Yassar.
Lithium batteries are what allow electric vehicles to travel several hundred miles on one charge. Their capacity for energy storage is well known, but so is their tendency to occasionally catch on fire – an occurrence known to battery researchers as “thermal runaway.” These fires occur most frequently when the batteries overheat or cycle rapidly. With more and more electric vehicles on the road each year, battery technology needs to adapt to reduce the likelihood of these dangerous and catastrophic fires.
Researchers from the University of Illinois at Chicago College of Engineering report that graphene — wonder material of the 21st century — may take the oxygen out of lithium battery fires. They report their findings in the journal Advanced Functional Materials.
The reasons lithium batteries catch fire include rapid cycling or charging and discharging, and high temperatures in the battery. These conditions can cause the cathode inside the battery — which in the case of most lithium batteries is a lithium-containing oxide, usually lithium cobalt oxide — to decompose and release oxygen. If the oxygen combines with other flammable products given off through decomposition of the electrolyte under high enough heat, spontaneous combustion can occur.
“We thought that if there was a way to prevent the oxygen from leaving the cathode and mixing with other flammable products in the battery, we could reduce the chances of a fire occurring,” said Reza Shahbazian-Yassar, associate professor of mechanical and industrial engineering in the UIC College of Engineering and corresponding author of the paper.
It turns out that a material Shahbazian-Yassar is very familiar with provided a perfect solution to this problem. That material is graphene — a super-thin layer of carbon atoms with unique properties. Shahbazian-Yassar and his colleagues previously had used graphene to help modulate lithium buildup on electrodes in lithium metal batteries.
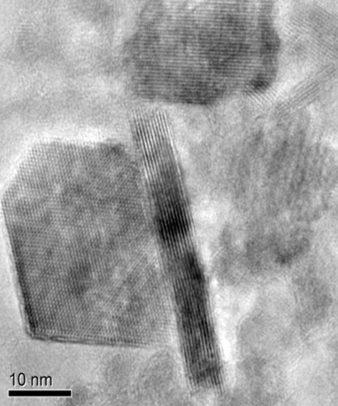
Shahbazian-Yassar and his colleagues knew that graphene sheets are impermeable to oxygen atoms. Graphene is also strong, flexible and can be made to be electrically conductive. Shahbazian-Yassar and Soroosh Sharifi-Asl, a graduate student in mechanical and industrial engineering at UIC and lead author of the paper, thought that if they wrapped very small particles of the lithium cobalt oxide cathode of a lithium battery in graphene, it might prevent oxygen from escaping.
First, the researchers chemically altered the graphene to make it electrically conductive. Next, they wrapped the tiny particles of lithium cobalt oxide cathode electrode in the conductive graphene.
When they looked at the graphene-wrapped lithium cobalt oxide particles using electron microscopy, they saw that the release of oxygen under high heat was reduced significantly compared with unwrapped particles.
Next, they bound together the wrapped particles with a binding material to form a usable cathode, and incorporated it into a lithium metal battery. When they measured released oxygen during battery cycling, they saw almost no oxygen escaping from cathodes even at very high voltages. The lithium metal battery continued to perform well even after 200 cycles.
“The wrapped cathode battery lost only about 14% of its capacity after rapid cycling compared to a conventional lithium metal battery where performance was down about 45% under the same conditions,” Sharifi-Asl said.
“Graphene is the ideal material for blocking the release of oxygen into the electrolyte,” Shahbazian-Yassar said. “It is impermeable to oxygen, electrically conductive, flexible, and is strong enough to withstand conditions within the battery. It is only a few nanometers thick so there would be no extra mass added to the battery. Our research shows that its use in the cathode can reliably reduce the release of oxygen and could be one way that the risk for fire in these batteries — which power everything from our phones to our cars — could be significantly reduced.”
###
Soroosh Sharifi-Asl, Tara Foroozan, Mohammad Asadi, Yifei Yuan, Ramasubramonian Deivanayagam, Ramin Rojaee, Boao Song and Amin Salehi-Khojin of the UIC College of Engineering; Fernando Soto and Perla Balbuena of Texas A & M University; and Xuanxuan Bi, Khalil Amine and Jun Lu of Argonne National Laboratory are co-authors on the paper.
This research was supported in part by the National Science Foundation award CMMI-1619743.
Media Contact
Sharon Parmet
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




