A groundbreaking study from the Picower Institute for Learning and Memory, in collaboration with the Alana Down Syndrome Center at MIT, ushers in a new era in the treatment of cognitive impairments associated with Down syndrome. Researchers have demonstrated that multi-sensory stimulation at the brain’s gamma frequency of 40Hz—delivered through light and sound—elicits remarkable improvements in cognition and neural connectivity in a mouse model genetically engineered to mimic Down syndrome. This discovery, detailed in a recent publication in PLOS ONE, represents the first evidence that such stimulation can enhance adult neurogenesis, potentially offering a non-invasive therapeutic strategy for a condition long considered intractable.
The method, known as Gamma Entrainment Using Sensory Stimulation (GENUS), leverages a rhythmic 40Hz sensory input designed to synchronize neuronal oscillations within the brain’s gamma frequency band. Gamma oscillations are rhythmic patterns of neural activity correlated with various facets of cognitive processing, including attention, memory, and sensory perception. Disruptions in these oscillations have been implicated in several neurological disorders, including Alzheimer’s disease and Down syndrome. By artificially entraining brain activity to this precise frequency, GENUS aims to restore normal network function and stimulate reparative processes at the cellular and molecular levels.
The study employed the Ts65Dn mouse model of Down syndrome, which, despite its limitations, replicates core pathological features of the human condition caused by trisomy 21. Male mice were subjected to daily one-hour sessions of synchronized 40Hz visual and auditory stimuli over a three-week period. Control groups were exposed to ambient light and sound but without rhythmic entrainment. Behavioral analyses revealed robust improvements in several standard short-term memory tasks, including tests designed to measure the recognition of novel objects and spatial navigation, functions heavily reliant on hippocampal integrity.
To elucidate the underlying neural changes responsible for these cognitive enhancements, the scientists performed detailed electrophysiological assessments. Increased neural activity was observed in the hippocampus of stimulated mice, suggesting that GENUS augments neuronal excitability and network engagement in regions crucial for memory encoding and retrieval. This evidence aligns with long-standing hypotheses that gamma frequency oscillations orchestrate communication within and among brain regions, facilitating the plasticity necessary for learning.
Complementing these functional measurements, the researchers undertook single-cell RNA sequencing—a high-resolution genomics technique that quantifies gene expression patterns in individual cells. This approach revealed striking shifts in the transcriptional landscape of hippocampal neurons in GENUS-treated subjects. Notably, there was moderated upregulation of synaptic genes linked with the formation and maintenance of neuronal connections, supporting the idea that gamma stimulation facilitates synaptogenesis and circuit remodeling underlying cognitive recovery.
Further microscopic analysis confirmed these molecular findings. Specifically, an increase in synaptic density was documented in the dentate gyrus, a hippocampal subregion implicated in memory formation and known to undergo neurogenesis throughout adulthood. The proliferation of synapses here is a potent anatomical substrate through which GENUS may exert its cognitive benefits. Such synaptic remodeling suggests improved synaptic transmission and network efficiency as functional correlates of sensory entrainment.
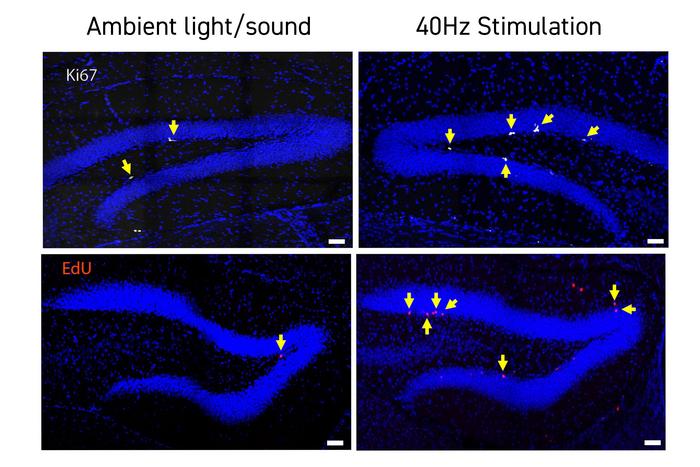
A profound observation emerged from the study’s genomic analyses: the modulation of TCF4 expression. TCF4 is a pivotal transcription factor involved in neurodevelopment and adult neurogenesis, processes that are compromised in Down syndrome. GENUS stimulation led to a partial restoration of TCF4 levels in hippocampal neurons, suggesting that rhythmic sensory entrainment can influence gene regulatory networks critical for neuronal birth and differentiation. The consequent surge in adult neurogenesis, confirmed through increased markers of newly generated neurons, may contribute significantly to enhanced plasticity and cognitive function.
This is the first study to link gamma frequency sensory stimulation directly to increased neurogenesis in a neurodevelopmental disorder model. By promoting the generation of new neurons in the adult brain, GENUS could potentially counteract neurogenic deficits inherent in Down syndrome, charting a path toward durable cognitive improvement. Although the exact causal mechanisms remain to be fully elucidated, the correlation between enhanced neurogenesis, improved synaptic connectivity, and better memory performance forms a compelling narrative.
Importantly, the study also shed light on neuroprotective effects associated with GENUS stimulation. Mice receiving 40Hz entrainment exhibited greater retention of neurons expressing Reelin, a protein implicated in synaptic plasticity, cognitive resilience, and neural migration. Since Reelin expression diminishes in Alzheimer’s disease—a condition with an alarmingly high prevalence in individuals with Down syndrome—its preservation by GENUS hints at a mechanism for delaying or mitigating neurodegeneration and related cognitive decline.
Despite these promising outcomes, the authors caution that the Ts65Dn mouse model does not fully replicate the human Down syndrome condition, particularly as the mice used in the study were all male. Moreover, the cognitive assessments focused solely on short-term memory enhancements, leaving open questions about long-term effects and generalizability to other cognitive domains. The research also concentrated on hippocampal changes, without examining potential impacts on other critical areas such as the prefrontal cortex, which governs executive function and decision-making.
Nevertheless, the translational potential of GENUS warrants serious consideration. Given that this non-invasive sensory stimulation can be delivered with relative ease and minimal risk, it presents a promising adjunct or alternative to pharmacological interventions that often carry significant side effects. Continuing clinical investigations, including early-phase trials at MIT with human volunteers, aim to determine whether these preclinical findings can be replicated and extended in people with Down syndrome, potentially paving the way for new therapies targeting cognitive deficits.
The co-corresponding authors, Li-Huei Tsai and postdoctoral researcher Md Rezaul Islam, emphasize the need for cautious optimism. While the results demonstrate a remarkable plasticity and restorative capacity within the brain, they underscore that much remains to be learned about dosing parameters, long-term outcomes, sex differences, and the interplay between gamma stimulation and the complex neuropathology of Down syndrome. Importantly, ongoing collaborations with bioengineering and clinical teams aim to optimize stimulation protocols and better understand the molecular cascades downstream of GENUS.
This study adds valuable insight to a growing body of research demonstrating the therapeutic potential of entraining brain rhythms to address a spectrum of neurological disorders. Prior work has shown GENUS benefits in Alzheimer’s disease models, stroke recovery, and chemotherapy-induced cognitive impairment. Its application to Down syndrome broadens the scope of brain stimulation technologies and opens fresh avenues for combating cognitive disabilities by harnessing intrinsic brain oscillations.
As our understanding of the dynamic relationships between neural oscillations, gene expression, and synaptic plasticity deepens, interventions like GENUS may revolutionize neurotherapeutics. By synchronizing the brain’s natural rhythms, this approach revitalizes aging or diseased circuits, encouraging regeneration and functional renewal at a fundamental level. For individuals and families affected by Down syndrome, these findings provide a beacon of hope for enhancing quality of life and cognitive potential through innovative, non-pharmacological means.
Subject of Research: Animals
Article Title: Multisensory gamma stimulation enhances adult neurogenesis and improves cognitive function in male mice with Down Syndrome
News Publication Date: 24-Apr-2025
Web References:
http://dx.doi.org/10.1371/journal.pone.0317428
Image Credits: Alana Down Syndrome Center at MIT
Keywords: Down syndrome, Neuroscience, Brain, Cognition, Neurology, Brain stimulation
Tags: 40Hz gamma frequency stimulationcellular reparative processes in the braincognitive impairments Down syndrome treatmentGamma Entrainment Using Sensory StimulationMIT Alana Down Syndrome Center collaboration.multi-sensory stimulation researchneural connectivity improvement techniquesneurogenesis enhancement in miceneurological disorders and gamma oscillationsneuronal oscillations and cognitionnon-invasive therapies for Down syndromerhythmic sensory input effects