Credit: Lou Group/Rice University
Scientists at Rice University have discovered that an atom-thick material being eyed for flexible electronics and next-generation optical devices is more brittle than they expected.
The Rice team led by materials scientist Jun Lou tested the tensile strength of two-dimensional, semiconducting molybdenum diselenide and discovered that flaws as small as one missing atom can initiate catastrophic cracking under strain.
The team's report appears this month in Advanced Materials.
The finding may cause industry to look more carefully at the properties of 2-D materials before incorporating them in new technologies, he said.
"It turns out not all 2-D crystals are equal," said Lou, a Rice professor of materials science and nanoengineering. "Graphene is a lot more robust compared with some of the others we're dealing with right now, like this molybdenum diselenide. We think it has something to do with defects inherent to these materials."
The defects could be as small as a single atom that leaves a vacancy in the crystalline structure, he said. "It's very hard to detect them," he said. "Even if a cluster of vacancies makes a bigger hole, it's difficult to find using any technique. It might be possible to see them with a transmission electron microscope, but that would be so labor-intensive that it wouldn't be useful."
Molybdenum diselenide is a dichalcogenide, a two-dimensional semiconducting material that appears as a graphene-like hexagonal array from above but is actually a sandwich of metallic atoms between two layers of chalcogen atoms, in this case, selenium. Molybdenum diselenide is being considered for use as transistors and in next-generation solar cells, photodetectors and catalysts as well as electronic and optical devices.
Lou and colleagues measured the material's elastic modulus, the amount of stretching a material can handle and still return to its initial state, at 177.2 (plus or minus 9.3) gigapascals. Graphene is more than five times as elastic. They attributed the large variation to pre-existing flaws of between 3.6 and 77.5 nanometers.
Its fracture strength, the amount of stretching a material can handle before breaking, was measured at 4.8 (plus or minus 2.9) gigapascals. Graphene is nearly 25 times stronger.
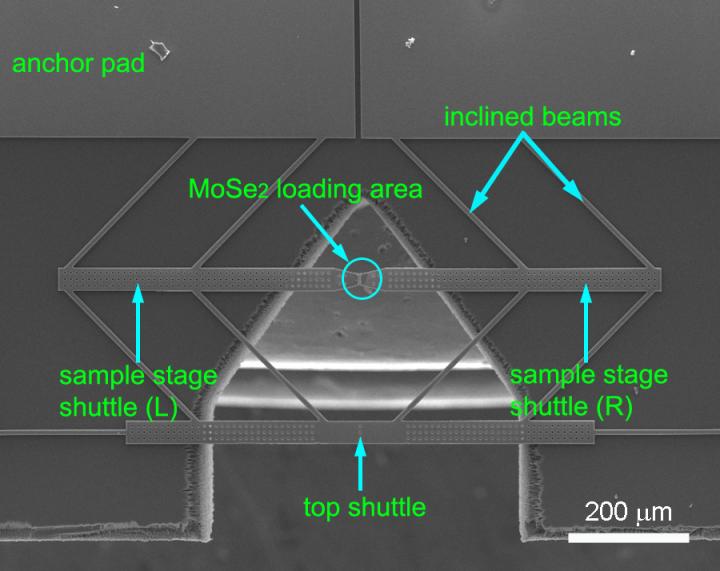
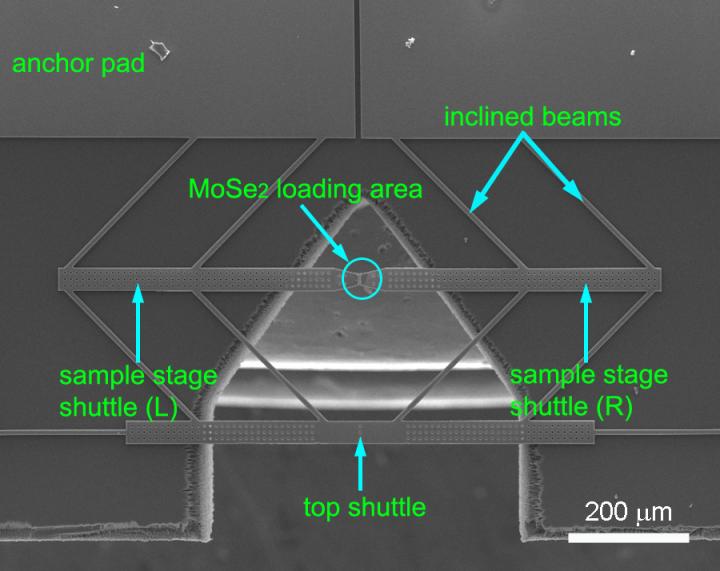
Part of the project led by Rice postdoctoral researcher Yingchao Yang required moving molybdenum diselenide from a growth chamber in a chemical vapor deposition furnace to a microscope without introducing more defects. Yang solved the problem using a dry transfer process in place of a standard acid washing that would have ruined the samples.
To test samples, Yang placed rectangles of molybdenum diselenide onto a sensitive electron microscope platform invented by the Lou group. Natural van der Waals forces held the samples in place on springy cantilever arms that measured the applied stress.
Lou said the group attempted to measure the material's fracture toughness, an indicator of how likely cracks are to propagate, as they had in an earlier study on graphene. But they found that pre-cutting cracks into molybdenum diselenide resulted in it shattering before stress could be applied, he said.
"The important message of this work is the brittle nature of these materials," Lou said. "A lot of people are thinking about using 2-D crystals because they're inherently thin. They're thinking about flexible electronics because they are semiconductors and their theoretical elastic strength should be very high. According to our calculations, they can be stretched up to 10 percent.
"But in reality, because of the inherent defects, you rarely can achieve that much strength. The samples we have tested so far broke at 2 to 3 percent (of the theoretical maximum) at most," Lou said. "That should still be fine for most flexible applications, but unless they find a way to quench the defects, it will be very hard to achieve the theoretical limits."
###
Co-authors of the paper are Rice graduate students Emily Hacopian, Weibing Chen, Jing Zhang and Bo Li, alumna Yongji Gong and Pulickel Ajayan, chair of Rice's Department of Materials Science and NanoEngineering, the Benjamin M. and Mary Greenwood Anderson Professor in Engineering and a professor of chemistry; Xing Li of Rice and Peking University, China; Minru Wen of Tsinghua University, China, and the Georgia Institute of Technology; Wu Zhou of Oak Ridge National Laboratory, Oak Ridge, Tenn.; Qing Chen of Peking University; and Ting Zhu of the Georgia Institute of Technology.
The research was supported by the Air Force Office of Scientific Research, the Welch Foundation, the Department of Energy Office of Basic Energy Sciences, the National Science Foundation and the National Science Foundation of China.
Read the abstract at http://onlinelibrary.wiley.com/doi/10.1002/adma.201604201/abstract
This news release can be found online at http://news.rice.edu/2016/11/14/2-d-material-a-brittle-surprise/
Follow Rice News and Media Relations via Twitter @RiceUNews
Related materials:
Lou Group: http://n3lab.rice.edu
Department of Materials Science and NanoEngineering: http://msne.rice.edu
George R. Brown School of Engineering: http://engr.rice.edu
Video: https://youtu.be/uvhyi5TwZXA
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,910 undergraduates and 2,809 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for happiest students and for lots of race/class interaction by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger's Personal Finance. To read "What they're saying about Rice," go to http://tinyurl.com/RiceUniversityoverview.
Media Contact
David Ruth
[email protected]
713-348-6327
@RiceUNews
http://news.rice.edu
############
Story Source: Materials provided by Scienmag